This is a preprint.
Seeding competent TDP-43 persists in human patient and mouse muscle
- PMID: 38617354
- PMCID: PMC11014586
- DOI: 10.1101/2024.04.03.587918
Seeding competent TDP-43 persists in human patient and mouse muscle
Update in
-
Seeding-competent TDP-43 persists in human patient and mouse muscle.Sci Transl Med. 2024 Nov 27;16(775):eadp5730. doi: 10.1126/scitranslmed.adp5730. Epub 2024 Nov 27. Sci Transl Med. 2024. PMID: 39602508 Free PMC article.
Abstract
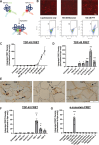
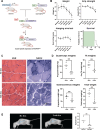
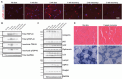
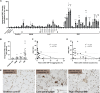
TAR DNA-binding protein 43 (TDP-43) is an RNA binding protein that accumulates as aggregates in the central nervous system of some neurodegenerative diseases. However, TDP-43 aggregation is also a sensitive and specific pathologic feature found in a family of degenerative muscle diseases termed inclusion body myopathy (IBM). TDP-43 aggregates from ALS and FTD brain lysates may serve as self-templating aggregate seeds in vitro and in vivo, supporting a prion-like spread from cell to cell. Whether a similar process occurs in IBM patient muscle is not clear. We developed a mouse model of inducible, muscle-specific cytoplasmic localized TDP-43. These mice develop muscle weakness with robust accumulation of insoluble and phosphorylated sarcoplasmic TDP-43, leading to eosinophilic inclusions, altered proteostasis and changes in TDP-43-related RNA processing that resolve with the removal of doxycycline. Skeletal muscle lysates from these mice also have seeding competent TDP-43, as determined by a FRET-based biosensor, that persists for weeks upon resolution of TDP-43 aggregate pathology. Human muscle biopsies with TDP-43 pathology also contain TDP-43 aggregate seeds. Using lysates from muscle biopsies of patients with IBM, IMNM and ALS we found that TDP-43 seeding capacity was specific to IBM. Surprisingly, TDP-43 seeding capacity anti-correlated with TDP-43 aggregate and vacuole abundance. These data support that TDP-43 aggregate seeds are present in IBM skeletal muscle and represent a unique TDP-43 pathogenic species not previously appreciated in human muscle disease.
Conflict of interest statement
Competing interests: None to disclose.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
