Characterization of the unique oral microbiome of children harboring Helicobacter pylori in the oral cavity
- PMID: 38617439
- PMCID: PMC11011227
- DOI: 10.1080/20002297.2024.2339158
Characterization of the unique oral microbiome of children harboring Helicobacter pylori in the oral cavity
Abstract
Objective: Helicobacter pylori infection is acquired in childhood via the oral cavity, although its relationship with the characteristics of the oral microbiome has not been elucidated. In this study, we performed comprehensive analysis of the oral microbiome in children and adults with or without H. pylori in the oral cavity.
Methods: Bacterial DNA was extracted from 41 adult and 21 child saliva specimens, and H. pylori was detected using PCR. 16S rRNA gene amplification was performed for next-generation sequencing. Bioinformatic analyses were conducted using Quantitative Insights into Microbial Ecology 2 (QIIME 2).
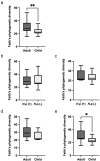
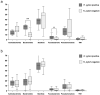
Results: Faith's phylogenetic diversity analysis showed a significant difference between H. pylori-negative adult and child specimens in terms of α-diversity (p < 0.05), while no significant difference was observed between H. pylori-positive adult and child specimens. There was also a significant difference in β-diversity between H. pylori-positive and negative child specimens (p < 0.05). Taxonomic analysis at the genus level revealed that Porphyromonas was the only bacterium that was significantly more abundant in both H. pylori-positive adults and children than in corresponding negative specimens (p < 0.01 and p < 0.05, respectively).
Conclusion: These results suggest unique oral microbiome characteristics in children with H. pylori infection in the oral cavity.
Keywords: Helicobacter pylori; metagenomic analysis; oral cavity; oral microbiome; saliva.
© 2024 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Analysis of by high-throughput sequencing: Helicobacter pylori infection and salivary microbiome.BMC Oral Health. 2020 Mar 20;20(1):84. doi: 10.1186/s12903-020-01070-1. BMC Oral Health. 2020. PMID: 32197614 Free PMC article.
-
The investigation of Helicobacter pylori in the dental biofilm and saliva samples of children with dyspeptic complaints.BMC Oral Health. 2017 Mar 21;17(1):67. doi: 10.1186/s12903-017-0361-x. BMC Oral Health. 2017. PMID: 28327128 Free PMC article.
-
Characterization of gut microbiome and metabolome in Helicobacter pylori patients in an underprivileged community in the United States.World J Gastroenterol. 2021 Sep 7;27(33):5575-5594. doi: 10.3748/wjg.v27.i33.5575. World J Gastroenterol. 2021. PMID: 34588753 Free PMC article.
-
DNA diagnostics for reliable and universal identification of Helicobacter pylori.World J Gastroenterol. 2021 Nov 7;27(41):7100-7112. doi: 10.3748/wjg.v27.i41.7100. World J Gastroenterol. 2021. PMID: 34887630 Free PMC article. Review.
-
Helicobacter pylori Infection, the Gastric Microbiome and Gastric Cancer.Adv Exp Med Biol. 2019;1149:195-210. doi: 10.1007/5584_2019_366. Adv Exp Med Biol. 2019. PMID: 31016631 Review.
Cited by
-
Oral Microbiota and the Risk of Gastrointestinal Cancers-A Narrative Literature Review.Pathogens. 2024 Sep 23;13(9):819. doi: 10.3390/pathogens13090819. Pathogens. 2024. PMID: 39339011 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
