A phase Ib/II trial of capmatinib plus spartalizumab vs. spartalizumab alone in patients with pretreated hepatocellular carcinoma
- PMID: 38617599
- PMCID: PMC11009449
- DOI: 10.1016/j.jhepr.2024.101021
A phase Ib/II trial of capmatinib plus spartalizumab vs. spartalizumab alone in patients with pretreated hepatocellular carcinoma
Abstract
Background & aims: This phase Ib/II trial evaluated the safety and efficacy of capmatinib in combination with spartalizumab or spartalizumab alone in patients with advanced hepatocellular carcinoma (HCC).
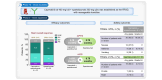
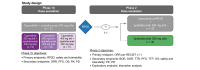
Methods: Eligible patients who had progressed or were intolerant to sorafenib received escalating doses of capmatinib 200 mg, 300 mg, and 400 mg twice a day (bid) plus spartalizumab 300 mg every 3 weeks (q3w) in the phase Ib study. Once the recommended phase II dose (RP2D) was determined, the phase II study commenced with randomised 1:1 treatment with either capmatinib + spartalizumab (n = 32) or spartalizumab alone (n = 30). Primary endpoints were safety and tolerability (phase Ib) and investigator-assessed overall response rate per RECIST v1.1 for combination vs. single-agent arms using a Bayesian logistic regression model (phase II).
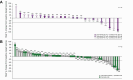
Results: In phase Ib, the RP2D for capmatinib in combination with spartalizumab was determined to be 400 mg bid. Dose-limiting toxicity consisting of grade 3 diarrhoea was reported in one patient at the capmatinib 400 mg bid + spartalizumab 300 mg q3w dose level. The primary endpoint in the phase II study was not met. The observed overall response rate in the capmatinib + spartalizumab arm was 9.4% vs. 10% in the spartalizumab arm. The most common any-grade treatment-related adverse events (TRAEs, ≥20%) were nausea (37.5%), asthenia and vomiting (28.1% each), diarrhoea, pyrexia, and decreased appetite (25.0% each) in the combination arm; TRAEs ≥10% were pruritus (23.3%), and rash (10.0%) in the spartalizumab-alone arm.
Conclusion: Capmatinib at 400 mg bid plus spartalizumab 300 mg q3w was established as the RP2D, with manageable toxicities and no significant safety signals, but the combination did not show superior clinical activity compared with spartalizumab single-agent treatment in patients with advanced HCC who had previously been treated with sorafenib.
Impact and implications: Simultaneous targeting of MET and programmed cell death protein 1 may provide synergistic clinical benefit in patients with advanced HCC. This is the first trial to report a combination of capmatinib (MET inhibitor) and spartalizumab (programmed cell death protein 1 inhibitor) as second-line treatment after sorafenib for advanced HCC. The combination did not show superior clinical activity compared with spartalizumab single-agent treatment in patients with advanced HCC who had previously been treated with sorafenib. The results indicate that there is a clear need to identify a reliable predictive marker of response for HCC and to identify patients with HCC that would benefit from the combination of checkpoint inhibitor +/- targeted therapy.
Clinical trial number: NCT02795429.
Keywords: Capmatinib; Hepatocellular carcinoma; MET; Spartalizumab; anti-programmed death-ligand 1.
© 2024 The Authors.
Conflict of interest statement
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Benson A.B., D’Angelica M.I., Abbott D.E., et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541–565. - PubMed
-
- Liver EAFTSOT EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
-
- Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

