Asphaltene Adsorption on Solid Surfaces Investigated Using Quartz Crystal Microbalance with Dissipation under Flow Conditions
- PMID: 38617650
- PMCID: PMC11007691
- DOI: 10.1021/acsomega.3c09294
Asphaltene Adsorption on Solid Surfaces Investigated Using Quartz Crystal Microbalance with Dissipation under Flow Conditions
Abstract
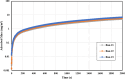
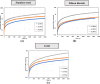
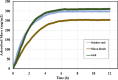
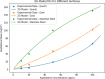
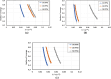
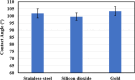
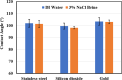
Asphaltenes can cause operational challenges in petroleum production facilities and adversely affect production by adsorption on mineral surfaces and alteration of the oil wettability of reservoirs. Therefore, understanding asphaltene adsorption mechanisms and their effects is crucial to improving the efficiency of oil production and reducing costs. In this study, we focus on understanding the impact of asphaltene concentration and the depositing environment of asphaltene adsorption on solid surfaces using the quartz crystal microbalance with dissipation (QCM-D) technique. The initial and long-term kinetics of adsorption at different concentrations were examined on three different solid surfaces including silicon dioxide to represent quartz mineral, stainless steel, and gold. The frequency-dissipation data showed evidence of monolayer adsorption initially, followed by multilayer formation. At short times, the adsorbed mass increased linearly with time, suggesting that the process was kinetically controlled rather than diffusion-controlled. The results were reproducible and did not depend on convection velocity but did depend on the surface material. At later stages, the monolayer development appeared to follow the random sequential adsorption (RSA) theory. Once multilayer adsorption commenced, the rates agreed well with the two-layer model of Zhu and Gu, 1990. The impact of asphaltene adsorption on the wettability of the surface was examined using contact angle studies, which showed decreasing water wettability with an increase in the adsorbed mass. The contact angle of water after 12 h of adsorption leveled off at around 100° on all three surfaces. Contact angle measurements were also used to evaluate if brine salinity causes the wettability alteration of surfaces with the adsorbed asphaltene. The results indicate that at 3% NaCl solution, the contact angle decreased only slightly by less than 2°.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
An experimental study of the effects of bacteria on asphaltene adsorption and wettability alteration of dolomite and quartz.Sci Rep. 2023 Dec 6;13(1):21497. doi: 10.1038/s41598-023-48680-7. Sci Rep. 2023. PMID: 38057408 Free PMC article.
-
Direct Evidence of Salinity and pH Effects on the Interfacial Interactions of Asphaltene-Brine-Silica Systems.Molecules. 2020 Mar 8;25(5):1214. doi: 10.3390/molecules25051214. Molecules. 2020. PMID: 32182670 Free PMC article.
-
Asphaltene adsorption on quartz sand in the presence of pre-adsorbed water.J Colloid Interface Sci. 2016 Oct 15;480:137-145. doi: 10.1016/j.jcis.2016.07.014. Epub 2016 Jul 9. J Colloid Interface Sci. 2016. PMID: 27423129
-
Label-free, real-time interaction and adsorption analysis 2: quartz crystal microbalance.Methods Mol Biol. 2013;996:313-22. doi: 10.1007/978-1-62703-354-1_18. Methods Mol Biol. 2013. PMID: 23504432 Review.
-
A Review of Oil-Solid Separation and Oil-Water Separation in Unconventional Heavy Oil Production Process.Int J Mol Sci. 2022 Dec 21;24(1):74. doi: 10.3390/ijms24010074. Int J Mol Sci. 2022. PMID: 36613516 Free PMC article. Review.
References
-
- Mullins O. C.; Sabbah H.; Eyssautier J.; Pomerantz A. E.; Barré L.; Andrews A. B.; Zare R. N. Advances in asphaltene science and the Yen–Mullins model. Energy Fuels 2012, 26 (7), 3986–4003. 10.1021/ef300185p. - DOI
-
- Groenzin H.; Mullins O. C. Molecular size and structure of asphaltenes from various sources. Energy Fuels 2000, 14 (3), 677–684. 10.1021/ef990225z. - DOI
-
- Alimohammadi S.; Zendehboudi S.; James L. A comprehensive review of asphaltene deposition in petroleum reservoirs: Theory, challenges, and tips. Fuel 2019, 252, 753–791. 10.1016/j.fuel.2019.03.016. - DOI
LinkOut - more resources
Full Text Sources
