Genetic load and viability of a future restored northern white rhino population
- PMID: 38617823
- PMCID: PMC11009427
- DOI: 10.1111/eva.13683
Genetic load and viability of a future restored northern white rhino population
Abstract
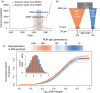
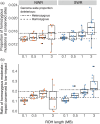
As biodiversity loss outpaces recovery, conservationists are increasingly turning to novel tools for preventing extinction, including cloning and in vitro gametogenesis of biobanked cells. However, restoration of populations can be hindered by low genetic diversity and deleterious genetic load. The persistence of the northern white rhino (Ceratotherium simum cottoni) now depends on the cryopreserved cells of 12 individuals. These banked genomes have higher genetic diversity than southern white rhinos (C. s. simum), a sister subspecies that successfully recovered from a severe bottleneck, but the potential impact of genetic load is unknown. We estimated how demographic history has shaped genome-wide genetic load in nine northern and 13 southern white rhinos. The bottleneck left southern white rhinos with more fixed and homozygous deleterious alleles and longer runs of homozygosity, whereas northern white rhinos retained more deleterious alleles masked in heterozygosity. To gauge the impact of genetic load on the fitness of a northern white rhino population restored from biobanked cells, we simulated recovery using fitness of southern white rhinos as a benchmark for a viable population. Unlike traditional restoration, cell-derived founders can be reintroduced in subsequent generations to boost lost genetic diversity and relieve inbreeding. In simulations with repeated reintroduction of founders into a restored population, the fitness cost of genetic load remained lower than that borne by southern white rhinos. Without reintroductions, rapid growth of the restored population (>20-30% per generation) would be needed to maintain comparable fitness. Our results suggest that inbreeding depression from genetic load is not necessarily a barrier to recovery of the northern white rhino and demonstrate how restoration from biobanked cells relieves some constraints of conventional restoration from a limited founder pool. Established conservation methods that protect healthy populations will remain paramount, but emerging technologies hold promise to bolster these tools to combat the extinction crisis.
Keywords: fitness; genetic load; genetic restoration; in vitro conservation; runs of homozygosity; simulation.
© 2024 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Altschul, S. F. , Gish, W. , Miller, W. , Myers, E. W. , & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410. - PubMed
-
- Armstrong, J. , Hickey, G. , Diekhans, M. , Fiddes, I. T. , Novak, A. M. , Deran, A. , Fang, Q. , Xie, D. , Feng, S. , Stiller, J. , Genereux, D. , Johnson, J. , Marinescu, V. D. , Alföldi, J. , Harris, R. S. , Lindblad‐Toh, K. , Haussler, D. , Karlsson, E. , Jarvis, E. D. , … Paten, B. (2020). Progressive cactus is a multiple‐genome aligner for the thousand‐genome era. Nature, 587(7833), 246–251. - PMC - PubMed
-
- Ashburner, M. , Ball, C. A. , Blake, J. A. , Botstein, D. , Butler, H. , Cherry, J. M. , Davis, A. P. , Dolinski, K. , Dwight, S. S. , Eppig, J. T. , Harris, M. A. , Hill, D. P. , Issel‐Tarver, L. , Kasarskis, A. , Lewis, S. , Matese, J. C. , Richardson, J. E. , Ringwald, M. , Rubin, G. M. , & Sherlock, G. (2000). Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nature Genetics, 25(1), 25–29. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

