Analysis of a Serious Adverse Reaction of Pulmonary Fibrosis Caused by Dronedarone
- PMID: 38617895
- PMCID: PMC11015333
- DOI: 10.1016/j.curtheres.2024.100743
Analysis of a Serious Adverse Reaction of Pulmonary Fibrosis Caused by Dronedarone
Abstract
Objective: This study aims to analyze a severe adverse reaction of pulmonary fibrosis induced by dronedarone hydrochloride tablets, and to provide a reference for clinical rational medication through drug precautions.
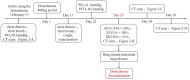
Methods: A case of pulmonary fibrosis induced by dronedarone hydrochloride tablets, along with related literature was retrospectively analyzed.
Results: Patients over 65 years old with a history of exposure to amiodarone may increase the incidence of pulmonary toxicity induced by dronedarone, and dronedarone should not be selected as a substitute treatment drug for patients with amiodarone-induced pulmonary toxicity.
Conclusions: It is recommended that clinicians monitor the diffusion capacity of carbon monoxide and lung ventilation function of patients before and after using dronedarone for treatment. For patients with a history of amiodarone exposure, intermittent monitoring of chest X-rays and lung function is necessary. If lung function decreases, dronedarone should be immediately discontinued.
Keywords: Adverse reactions; Amiodarone hydrochloride tablets; Dronedarone hydrochloride tablets; Drug precaution; Pulmonary fibrosis.
© 2024 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Stack S, Nguyen DV, Casto A, Ahuja N. Diffuse alveolar damage in a patient receiving dronedarone. Chest. 2015;147(4):e131–e133. - PubMed
-
- Hernández Voth AR, Catalán JS, Benavides Mañas PD, et al. A 73-year-old man with interstitial lung disease due to dronedarone. Am J Respir Crit Care Med. 2012;186(2):201–202. - PubMed
-
- Prescribing information for dronedarone. http://products.sanofi.us/multaq/multaq.html. Accessed September 18, 2013.
Publication types
LinkOut - more resources
Full Text Sources