Brillouin microscopy monitors rapid responses in subcellular compartments
- PMID: 38618142
- PMCID: PMC11006764
- DOI: 10.1186/s43074-024-00123-w
Brillouin microscopy monitors rapid responses in subcellular compartments
Abstract
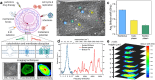
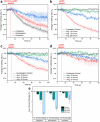
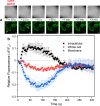
Measurements and imaging of the mechanical response of biological cells are critical for understanding the mechanisms of many diseases, and for fundamental studies of energy, signal and force transduction. The recent emergence of Brillouin microscopy as a powerful non-contact, label-free way to non-invasively and non-destructively assess local viscoelastic properties provides an opportunity to expand the scope of biomechanical research to the sub-cellular level. Brillouin spectroscopy has recently been validated through static measurements of cell viscoelastic properties, however, fast (sub-second) measurements of sub-cellular cytomechanical changes have yet to be reported. In this report, we utilize a custom multimodal spectroscopy system to monitor for the very first time the rapid viscoelastic response of cells and subcellular structures to a short-duration electrical impulse. The cytomechanical response of three subcellular structures - cytoplasm, nucleoplasm, and nucleoli - were monitored, showing distinct mechanical changes despite an identical stimulus. Through this pioneering transformative study, we demonstrate the capability of Brillouin spectroscopy to measure rapid, real-time biomechanical changes within distinct subcellular compartments. Our results support the promising future of Brillouin spectroscopy within the broad scope of cellular biomechanics.
Keywords: Brillouin scattering; Fluorescence; Imaging; Microscopy; Raman scattering.
© The Author(s) 2024.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov. 2019;9:837–851. doi: 10.1158/2159-8290.CD-19-0015. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
