Neonatal hypoglycaemia
- PMID: 38618170
- PMCID: PMC11015200
- DOI: 10.1136/bmjmed-2023-000544
Neonatal hypoglycaemia
Abstract
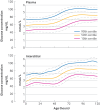
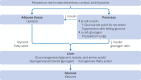
Low blood concentrations of glucose (hypoglycaemia) soon after birth are common because of the delayed metabolic transition from maternal to endogenous neonatal sources of glucose. Because glucose is the main energy source for the brain, severe hypoglycaemia can cause neuroglycopenia (inadequate supply of glucose to the brain) and, if severe, permanent brain injury. Routine screening of infants at risk and treatment when hypoglycaemia is detected are therefore widely recommended. Robust evidence to support most aspects of management is lacking, however, including the appropriate threshold for diagnosis and optimal monitoring. Treatment is usually initially more feeding, with buccal dextrose gel, followed by intravenous dextrose. In infants at risk, developmental outcomes after mild hypoglycaemia seem to be worse than in those who do not develop hypoglycaemia, but the reasons for these observations are uncertain. Here, the current understanding of the pathophysiology of neonatal hypoglycaemia and recent evidence regarding its diagnosis, management, and outcomes are reviewed. Recommendations are made for further research priorities.
Keywords: Endocrinology; Neonatology; Neuropathology; Perinatology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: none.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
