Disease-Modifying Treatments and Their Future in Alzheimer's Disease Management
- PMID: 38618323
- PMCID: PMC11014642
- DOI: 10.7759/cureus.56105
Disease-Modifying Treatments and Their Future in Alzheimer's Disease Management
Abstract
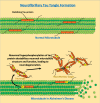
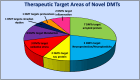
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by memory impairment, a loss of cholinergic neurons, and cognitive decline that insidiously progresses to dementia. The pathoetiology of AD is complex, as genetic predisposition, age, inflammation, oxidative stress, and dysregulated proteostasis all contribute to its development and progression. The histological hallmarks of AD are the formation and accumulation of amyloid-β plaques and interfibrillar tau tangles within the central nervous system. These histological hallmarks trigger neuroinflammation and disrupt the physiological structure and functioning of neurons, leading to cognitive dysfunction. Most treatments currently available for AD focus only on symptomatic relief. Disease-modifying treatments (DMTs) that target the biology of the disease in hopes of slowing or reversing disease progression are desperately needed. This narrative review investigates novel DMTs and their therapeutic targets that are either in phase three of development or have been recently approved by the U.S. Food and Drug Administration (FDA). The target areas of some of these novel DMTs consist of combatting amyloid or tau accumulation, oxidative stress, neuroinflammation, and dysregulated proteostasis, metabolism, or circadian rhythm. Neuroprotection and neuroplasticity promotion were also key target areas. DMT therapeutic target diversity may permit improved therapeutic responses in certain subpopulations of AD, particularly if the therapeutic target of the DMT being administered aligns with the subpopulation's most prominent pathological findings. Clinicians should be cognizant of how these novel drugs differ in therapeutic targets, as this knowledge may potentially enhance the level of care they can provide to AD patients in the future.
Keywords: alzheimers disease; anti-amyloid; anti-tau therapies; neuro inflammation; neurodegenerative disesase.
Copyright © 2024, Smith et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
A Recent Update on Pathophysiology and Therapeutic Interventions of Alzheimer's Disease.Curr Pharm Des. 2023;29(43):3428-3441. doi: 10.2174/0113816128264355231121064704. Curr Pharm Des. 2023. PMID: 38038007 Review.
-
Immunosenescence and Aging: Neuroinflammation Is a Prominent Feature of Alzheimer's Disease and Is a Likely Contributor to Neurodegenerative Disease Pathogenesis.J Pers Med. 2022 Nov 2;12(11):1817. doi: 10.3390/jpm12111817. J Pers Med. 2022. PMID: 36579548 Free PMC article. Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Alzheimer's disease: Molecular aspects and treatment opportunities using herbal drugs.Ageing Res Rev. 2023 Jul;88:101960. doi: 10.1016/j.arr.2023.101960. Epub 2023 May 22. Ageing Res Rev. 2023. PMID: 37224884 Review.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
Cited by
-
Return of research results across the Alzheimer's Disease Research Centers network.Alzheimers Dement. 2025 Jun;21(6):e70418. doi: 10.1002/alz.70418. Alzheimers Dement. 2025. PMID: 40545555 Free PMC article.
-
Brain Glucose Hypometabolism and Brain Iron Accumulation as Therapeutic Targets for Alzheimer's Disease and Other CNS Disorders.Pharmaceuticals (Basel). 2025 Feb 19;18(2):271. doi: 10.3390/ph18020271. Pharmaceuticals (Basel). 2025. PMID: 40006083 Free PMC article. Review.
-
Unlocking the Potential: Semaglutide's Impact on Alzheimer's and Parkinson's Disease in Animal Models.Curr Issues Mol Biol. 2024 Jun 13;46(6):5929-5949. doi: 10.3390/cimb46060354. Curr Issues Mol Biol. 2024. PMID: 38921025 Free PMC article. Review.
-
Exploring interdisciplinary perspectives on the implementation of personalized medicine and patient-orchestrated care in Alzheimer's disease: A qualitative study within the ABOARD research project.J Alzheimers Dis. 2025 May;105(1):120-133. doi: 10.1177/13872877251326166. Epub 2025 Mar 21. J Alzheimers Dis. 2025. PMID: 40116704 Free PMC article.
-
Alzheimer's Disease: Recent Developments in Pathogenesis, Diagnosis, and Therapy.Life (Basel). 2025 Mar 27;15(4):549. doi: 10.3390/life15040549. Life (Basel). 2025. PMID: 40283104 Free PMC article.
References
-
- Global health and aging. Suzman R, Beard J. https://www.nia.nih.gov/sites/default/files/2017-06/global_health_aging.pdf NIH. 2011;11:7737.
-
- Dementia in 2014. Towards early diagnosis in Alzheimer disease. Nordberg A. Nat Rev Neurol. 2015;11:69–70. - PubMed
-
- Alzheimer's disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Monteiro AR, Barbosa DJ, Remião F, Silva R. Biochem Pharmacol. 2023;211:115522. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
