Efficacy of genotype-matched vaccine against re-emerging genotype V Japanese encephalitis virus
- PMID: 38618740
- PMCID: PMC11060017
- DOI: 10.1080/22221751.2024.2343910
Efficacy of genotype-matched vaccine against re-emerging genotype V Japanese encephalitis virus
Abstract
Japanese encephalitis (JE), caused by the Japanese encephalitis virus (JEV), is a highly threatening disease with no specific treatment. Fortunately, the development of vaccines has enabled effective defense against JE. However, re-emerging genotype V (GV) JEV poses a challenge as current vaccines are genotype III (GIII)-based and provide suboptimal protection. Given the isolation of GV JEVs from Malaysia, China, and the Republic of Korea, there is a concern about the potential for a broader outbreak. Under the hypothesis that a GV-based vaccine is necessary for effective defense against GV JEV, we developed a pentameric recombinant antigen using cholera toxin B as a scaffold and mucosal adjuvant, which was conjugated with the E protein domain III of GV by genetic fusion. This GV-based vaccine antigen induced a more effective immune response in mice against GV JEV isolates compared to GIII-based antigen and efficiently protected animals from lethal challenges. Furthermore, a bivalent vaccine approach, inoculating simultaneously with GIII- and GV-based antigens, showed protective efficacy against both GIII and GV JEVs. This strategy presents a promising avenue for comprehensive protection in regions facing the threat of diverse JEV genotypes, including both prevalent GIII and GI as well as emerging GV strains.
Keywords: Japanese encephalitis virus; genotype V; neutralizing antibody; recombinant vaccine; bivalent vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
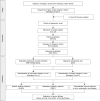
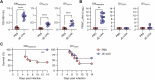

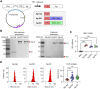
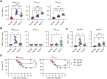
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
