Serious Bleeding in Patients With Atrial Fibrillation Using Diltiazem With Apixaban or Rivaroxaban
- PMID: 38619832
- PMCID: PMC11019444
- DOI: 10.1001/jama.2024.3867
Serious Bleeding in Patients With Atrial Fibrillation Using Diltiazem With Apixaban or Rivaroxaban
Abstract
Importance: Diltiazem, a commonly prescribed ventricular rate-control medication for patients with atrial fibrillation, inhibits apixaban and rivaroxaban elimination, possibly causing overanticoagulation.
Objective: To compare serious bleeding risk for new users of apixaban or rivaroxaban with atrial fibrillation treated with diltiazem or metoprolol.
Design, setting, and participants: This retrospective cohort study included Medicare beneficiaries aged 65 years or older with atrial fibrillation who initiated apixaban or rivaroxaban use and also began treatment with diltiazem or metoprolol between January 1, 2012, and November 29, 2020. Patients were followed up to 365 days through November 30, 2020. Data were analyzed from August 2023 to February 2024.
Exposures: Diltiazem and metoprolol.
Main outcomes and measures: The primary outcome was a composite of bleeding-related hospitalization and death with recent evidence of bleeding. Secondary outcomes were ischemic stroke or systemic embolism, major ischemic or hemorrhagic events (ischemic stroke, systemic embolism, intracranial or fatal extracranial bleeding, or death with recent evidence of bleeding), and death without recent evidence of bleeding. Hazard ratios (HRs) and rate differences (RDs) were adjusted for covariate differences with overlap weighting.
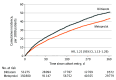
Results: The study included 204 155 US Medicare beneficiaries, of whom 53 275 received diltiazem and 150 880 received metoprolol. Study patients (mean [SD] age, 76.9 [7.0] years; 52.7% female) had 90 927 person-years (PY) of follow-up (median, 120 [IQR, 59-281] days). Patients receiving diltiazem treatment had increased risk for the primary outcome (RD, 10.6 [95% CI, 7.0-14.2] per 1000 PY; HR, 1.21 [95% CI, 1.13-1.29]) and its components of bleeding-related hospitalization (RD, 8.2 [95% CI, 5.1-11.4] per 1000 PY; HR, 1.22 [95% CI, 1.13-1.31]) and death with recent evidence of bleeding (RD, 2.4 [95% CI, 0.6-4.2] per 1000 PY; HR, 1.19 [95% CI, 1.05-1.34]) compared with patients receiving metoprolol. Risk for the primary outcome with initial diltiazem doses exceeding 120 mg/d (RD, 15.1 [95% CI, 10.2-20.1] per 1000 PY; HR, 1.29 [95% CI, 1.19-1.39]) was greater than that for lower doses (RD, 6.7 [95% CI, 2.0-11.4] per 1000 PY; HR, 1.13 [95% CI, 1.04-1.24]). For doses exceeding 120 mg/d, the risk of major ischemic or hemorrhagic events was increased (HR, 1.14 [95% CI, 1.02-1.27]). Neither dose group had significant changes in the risk for ischemic stroke or systemic embolism or death without recent evidence of bleeding. When patients receiving high- and low-dose diltiazem treatment were directly compared, the HR for the primary outcome was 1.14 (95% CI, 1.02-1.26).
Conclusions and relevance: In Medicare patients with atrial fibrillation receiving apixaban or rivaroxaban, diltiazem was associated with greater risk of serious bleeding than metoprolol, particularly for diltiazem doses exceeding 120 mg/d.
Conflict of interest statement
Figures


Comment in
-
Bleeding in Patients With Atrial Fibrillation.JAMA. 2024 Sep 17;332(11):933-934. doi: 10.1001/jama.2024.13789. JAMA. 2024. PMID: 39158905 No abstract available.
References
-
- Xarelto. Prescribing information. Janssen; 2019. Accessed February 13, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215859s000lbl.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

