Neurological diagnoses in hospitalized COVID-19 patients associated with adverse outcomes: A multinational cohort study
- PMID: 38620037
- PMCID: PMC11018281
- DOI: 10.1371/journal.pdig.0000484
Neurological diagnoses in hospitalized COVID-19 patients associated with adverse outcomes: A multinational cohort study
Erratum in
-
Correction: Neurological diagnoses in hospitalized COVID-19 patients associated with adverse outcomes: A multinational cohort study.PLOS Digit Health. 2025 Jul 22;4(7):e0000957. doi: 10.1371/journal.pdig.0000957. eCollection 2025 Jul. PLOS Digit Health. 2025. PMID: 40694524 Free PMC article.
Abstract
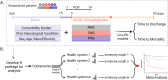
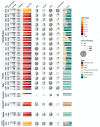
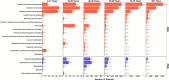
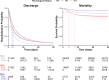
Few studies examining the patient outcomes of concurrent neurological manifestations during acute COVID-19 leveraged multinational cohorts of adults and children or distinguished between central and peripheral nervous system (CNS vs. PNS) involvement. Using a federated multinational network in which local clinicians and informatics experts curated the electronic health records data, we evaluated the risk of prolonged hospitalization and mortality in hospitalized COVID-19 patients from 21 healthcare systems across 7 countries. For adults, we used a federated learning approach whereby we ran Cox proportional hazard models locally at each healthcare system and performed a meta-analysis on the aggregated results to estimate the overall risk of adverse outcomes across our geographically diverse populations. For children, we reported descriptive statistics separately due to their low frequency of neurological involvement and poor outcomes. Among the 106,229 hospitalized COVID-19 patients (104,031 patients ≥18 years; 2,198 patients <18 years, January 2020-October 2021), 15,101 (14%) had at least one CNS diagnosis, while 2,788 (3%) had at least one PNS diagnosis. After controlling for demographics and pre-existing conditions, adults with CNS involvement had longer hospital stay (11 versus 6 days) and greater risk of (Hazard Ratio = 1.78) and faster time to death (12 versus 24 days) than patients with no neurological condition (NNC) during acute COVID-19 hospitalization. Adults with PNS involvement also had longer hospital stay but lower risk of mortality than the NNC group. Although children had a low frequency of neurological involvement during COVID-19 hospitalization, a substantially higher proportion of children with CNS involvement died compared to those with NNC (6% vs 1%). Overall, patients with concurrent CNS manifestation during acute COVID-19 hospitalization faced greater risks for adverse clinical outcomes than patients without any neurological diagnosis. Our global informatics framework using a federated approach (versus a centralized data collection approach) has utility for clinical discovery beyond COVID-19.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
All authors report no competing interests or conflicts of interest. JGK reports a consulting relationship with the i2b2-tranSMART Foundation through Invocate, Inc. RB reports being a shareholder of Biomeris s.r.l. and Engenome s.r.l. DAH reports entitled to royalties from the University of Michigan for licensing of the EMERSE "synonyms". AM’s work is being funded by the Federal Ministry of Education and Research (BMBF) in Germany in the framework of the MIRACUM Consortium. AM reports being a shareholder of Biomeris s.r.l. BM reports being co-founder and equity owner from DESKI. DLM has received research support from the National Institutes of Health, Department of Veteran Affairs, and the University of Pittsburgh/Pittsburgh Health Data Alliance outside of this work. PA reports consulting for CCHMC and BCH. NG is a co-founder and equity owner of Datavisyn. ZX has served as a Consultant for Genentech/Roche. The institution of ZX has received research support from the National Institute of Health, the National Multiple Sclerosis Society, Food and Drug Administration, the Pittsburgh Foundation, the PNC Charitable Trust, the Ethel Vincent Trust, and Genentech / Roche.
Figures


References
Grants and funding
- UL1 TR001857/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- U24 CA210967/CA/NCI NIH HHS/United States
- R01 NS098023/NS/NINDS NIH HHS/United States
- R01 NS124882/NS/NINDS NIH HHS/United States
- U01 TR003528/TR/NCATS NIH HHS/United States
- P30 ES017885/ES/NIEHS NIH HHS/United States
- R01 LM013337/LM/NLM NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- FS/19/52/34563/BHF_/British Heart Foundation/United Kingdom
- T32 HD040128/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
