The mechanistic basis linking cytokine storm to thrombosis in COVID-19
- PMID: 38620974
- PMCID: PMC9116969
- DOI: 10.1016/j.tru.2022.100110
The mechanistic basis linking cytokine storm to thrombosis in COVID-19
Abstract
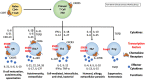
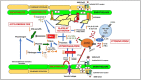
It is now well established that infection with SARS-CoV-2 resulting in COVID-19 disease includes a severely symptomatic subset of patients in whom an aggressive and/or dysregulated host immune response leads to cytokine storm syndrome (CSS) that may be further complicated by thrombotic events, contributing to the severe morbidity and mortality observed in COVID-19. This review provides a brief overview of cytokine storm in COVID-19, and then presents a mechanistic discussion of how cytokine storm affects integrated pathways in thrombosis involving the endothelium, platelets, the coagulation cascade, eicosanoids, auto-antibody mediated thrombosis, and the fibrinolytic system.
Keywords: COVID-19; Clots; Coagulopathy; Cytokine storm; SARS-CoV2; Thrombosis.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Hussell T., Bell T.J. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14(2):81–93. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
