Proof-of-concept studies with a computationally designed Mpro inhibitor as a synergistic combination regimen alternative to Paxlovid
- PMID: 38621119
- PMCID: PMC11046628
- DOI: 10.1073/pnas.2320713121
Proof-of-concept studies with a computationally designed Mpro inhibitor as a synergistic combination regimen alternative to Paxlovid
Abstract
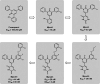
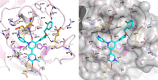
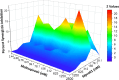
As the SARS-CoV-2 virus continues to spread and mutate, it remains important to focus not only on preventing spread through vaccination but also on treating infection with direct-acting antivirals (DAA). The approval of Paxlovid, a SARS-CoV-2 main protease (Mpro) DAA, has been significant for treatment of patients. A limitation of this DAA, however, is that the antiviral component, nirmatrelvir, is rapidly metabolized and requires inclusion of a CYP450 3A4 metabolic inhibitor, ritonavir, to boost levels of the active drug. Serious drug-drug interactions can occur with Paxlovid for patients who are also taking other medications metabolized by CYP4503A4, particularly transplant or otherwise immunocompromised patients who are most at risk for SARS-CoV-2 infection and the development of severe symptoms. Developing an alternative antiviral with improved pharmacological properties is critical for treatment of these patients. By using a computational and structure-guided approach, we were able to optimize a 100 to 250 μM screening hit to a potent nanomolar inhibitor and lead compound, Mpro61. In this study, we further evaluate Mpro61 as a lead compound, starting with examination of its mode of binding to SARS-CoV-2 Mpro. In vitro pharmacological profiling established a lack of off-target effects, particularly CYP450 3A4 inhibition, as well as potential for synergy with the currently approved alternate antiviral, molnupiravir. Development and subsequent testing of a capsule formulation for oral dosing of Mpro61 in B6-K18-hACE2 mice demonstrated favorable pharmacological properties, efficacy, and synergy with molnupiravir, and complete recovery from subsequent challenge by SARS-CoV-2, establishing Mpro61 as a promising potential preclinical candidate.
Keywords: Mpro61; SARS-CoV-2; drug synergy; molnupiravir; protease inhibitor.
Conflict of interest statement
Competing interests statement:Yale University has filed a patent application covering Mpro61.
Figures




Similar articles
-
Characteristics and Outcomes of US Veterans With Immunocompromised Conditions at High Risk of SARS-CoV-2 Infection With or Without Receipt of Oral Antiviral Agents.Clin Infect Dis. 2024 Feb 17;78(2):330-337. doi: 10.1093/cid/ciad504. Clin Infect Dis. 2024. PMID: 37619991
-
Genetic Surveillance of SARS-CoV-2 Mpro Reveals High Sequence and Structural Conservation Prior to the Introduction of Protease Inhibitor Paxlovid.mBio. 2022 Aug 30;13(4):e0086922. doi: 10.1128/mbio.00869-22. Epub 2022 Jul 13. mBio. 2022. PMID: 35862764 Free PMC article.
-
Editorial: Rebound COVID-19 and Cessation of Antiviral Treatment for SARS-CoV-2 with Paxlovid and Molnupiravir.Med Sci Monit. 2022 Oct 1;28:e938532. doi: 10.12659/MSM.938532. Med Sci Monit. 2022. PMID: 36181334 Free PMC article.
-
A tale of two drugs: Molnupiravir and Paxlovid.Mutat Res Rev Mutat Res. 2025 Jan-Jun;795:108533. doi: 10.1016/j.mrrev.2025.108533. Epub 2025 Feb 6. Mutat Res Rev Mutat Res. 2025. PMID: 39920989 Review.
-
[Nirmatrelvir plus ritonavir (Paxlovid) a potent SARS-CoV-2 3CLpro protease inhibitor combination].Rev Esp Quimioter. 2022 Jun;35(3):236-240. doi: 10.37201/req/002.2022. Epub 2022 Feb 21. Rev Esp Quimioter. 2022. PMID: 35183067 Free PMC article. Review. Spanish.
Cited by
-
From Detection to Protection: Antibodies and Their Crucial Role in Diagnosing and Combatting SARS-CoV-2.Vaccines (Basel). 2024 Apr 25;12(5):459. doi: 10.3390/vaccines12050459. Vaccines (Basel). 2024. PMID: 38793710 Free PMC article. Review.
-
Exploring Possible Drug-Resistant Variants of SARS-CoV-2 Main Protease (Mpro) with Noncovalent Preclinical Candidate, Mpro61.ACS Bio Med Chem Au. 2025 Jan 27;5(1):215-226. doi: 10.1021/acsbiomedchemau.4c00109. eCollection 2025 Feb 19. ACS Bio Med Chem Au. 2025. PMID: 39990941 Free PMC article.
-
Beta Spike-Presenting SARS-CoV-2 Virus-like Particle Vaccine Confers Broad Protection against Other VOCs in Mice.Vaccines (Basel). 2024 Sep 2;12(9):1007. doi: 10.3390/vaccines12091007. Vaccines (Basel). 2024. PMID: 39340037 Free PMC article.
-
The KT Jeang retrovirology prize 2024: Walther Mothes.Retrovirology. 2024 Oct 24;21(1):16. doi: 10.1186/s12977-024-00649-8. Retrovirology. 2024. PMID: 39449025 Free PMC article. No abstract available.
-
Structure-based discovery of highly bioavailable, covalent, broad-spectrum coronavirus MPro inhibitors with potent in vivo efficacy.Sci Adv. 2025 Apr 25;11(17):eadt7836. doi: 10.1126/sciadv.adt7836. Epub 2025 Apr 23. Sci Adv. 2025. PMID: 40267184 Free PMC article.
References
-
- Centers for Disease Control and Prevention, COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home (2023). Accessed 27 October 2023.
-
- Centers for Disease Control and Prevention, SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classificatio... (2023). Accessed 27 October 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

