Associations between change in BMI and the risk of hypertension and dyslipidaemia in people receiving integrase strand-transfer inhibitors, tenofovir alafenamide, or both compared with other contemporary antiretroviral regimens: a multicentre, prospective observational study from the RESPOND consortium cohorts
- PMID: 38621392
- PMCID: PMC11338627
- DOI: 10.1016/S2352-3018(23)00328-4
Associations between change in BMI and the risk of hypertension and dyslipidaemia in people receiving integrase strand-transfer inhibitors, tenofovir alafenamide, or both compared with other contemporary antiretroviral regimens: a multicentre, prospective observational study from the RESPOND consortium cohorts
Abstract
Background: Integrase strand-transfer inhibitors (INSTIs) and tenofovir alafenamide have been associated with weight gain in several clinical trials and observational cohorts. However, whether weight gain associated with INSTIs and tenofovir alafenamide confers a higher risk of weight-related clinical events is unclear. We aimed to assess whether changes in BMI differentially increase hypertension or dyslipidaemia risk in people with HIV receiving INSTIs, tenofovir alafenamide, or both versus other contemporary regimens.
Methods: This multicentre, prospective observational study analysed prospective data from RESPOND, an international consortium of HIV cohorts for which recruitment began in 2017 and is still ongoing from HIV clinics and hospitals in 37 European countries and Australia. Participants were eligible if they were aged 18 years or older, receiving INSTI-containing antiretroviral therapy (ART) regimens or a contemporary non-INSTI, did not have hypertension or dyslipidaemia at baseline, and had baseline and at least two follow-up BMI, lipid, and blood pressure measurements. We excluded participants without baseline CD4 or HIV RNA results and those receiving non-ART medications associated with weight changes, including antipsychotics and mood stabilisers, corticosteroids, insulin, and insulin secretagogues. They were followed up from baseline until the earliest hypertension or dyslipidaemia event, their last visit, or Dec 31, 2021, whichever was earlier. The primary outcomes were incidence of hypertension and dyslipidaemia, for which we used multivariable Poisson regression adjusted for time-updated BMI to determine unadjusted and adjusted incidence rate ratios (IRRs) of hypertension and dyslipidaemia in people receiving INSTIs, tenofovir alafenamide, or both, and tested for interaction between time-updated ART regimen and BMI.
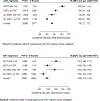
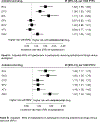
Findings: Of the 35 941 RESPOND participants, 9704 (7327 [75·5 %] male and 2377 [24·5%] female) were included in the hypertension analysis and 5231 (3796 [72·6%] male and 1435 [27·4%] female) were included in the dyslipidaemia analysis. In the univariable model, hypertension was more common in individuals receiving an INSTI with tenofovir alafenamide (IRR 1·70, 95% CI 1·54-1·88) or an INSTI without tenofovir alafenamide (1·41, 1·30-1·53) compared with those receiving neither INSTIs nor tenofovir alafenamide. Adjustment for time-updated BMI and confounders attenuated risk in participants receiving an INSTI with (IRR 1·48, 1·31-1·68) or without (1·25, 1·13-1·39) tenofovir alafenamide. Similarly, dyslipidaemia was more common in participants using tenofovir alafenamide with an INSTI (IRR 1·24, 1·10-1·40) and tenofovir alafenamide alone (1·22, 1·03-1·44) than in participants using neither INSTI nor tenofovir alafenamide. Adjustment for BMI and confounders attenuated the risk in participants receiving tenofovir alafenamide with an INSTI (adjusted IRR 1·21, 1·07-1·37), whereas the risk in those receiving tenofovir alafenamide alone became non-significant (1·15, 0·96-1·38). The associations between increasing BMI and risk of hypertension and dyslipidaemia did not differ between participants receiving different ART regimens (pinteraction=0·46 for hypertension; pinteraction=0·31 for dyslipidaemia).
Interpretation: Although residual confounding cannot be entirely excluded, the use of INSTIs was associated with incident hypertension, and the use of tenofovir alafenamide was associated with dyslipidaemia, with the latter association partly mediated by weight gain. These results reiterate the need for hypertension and dyslipidaemia screening in people with HIV.
Funding: The CHU St Pierre Brussels HIV Cohort, The Austrian HIV Cohort Study, The Australian HIV Observational Database, The AIDS Therapy Evaluation in the Netherlands national observational HIV cohort, The Brighton HIV Cohort, The National Croatian HIV Cohort, The EuroSIDA cohort, The Frankfurt HIV Cohort Study, The Georgian National AIDS Health Information System, The Nice HIV Cohort, The ICONA Foundation, The Modena HIV Cohort, The PISCIS Cohort Study, The Swiss HIV Cohort Study, The Swedish InfCare HIV Cohort, The Royal Free HIV Cohort Study, The San Raffaele Scientific Institute, The University Hospital Bonn HIV Cohort, The University of Cologne HIV Cohort, Merck Life Sciences, ViiV Healthcare, and Gilead Sciences.
© 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests AM has received travel support and lecture and consultancy fees from Gilead Sciences, ViiV Healthcare, Eiland, and Bonnin, all outside the submitted work. VV is an employee of and has stocks in ViiV Healthcare. CC is an employee of and has stocks in Gilead Sciences. EB is an employee of and has stocks in MSD. ML has received sitting fees from Certa Therapeutics data safety monitoring board. KG-P has served on advisory boards and provided lectures for Gilead Sciences and ViiV Healthcare. AR has received support for attending meetings and travel from Gilead Sciences and Pfizer, received an investigator-initiated trial grant from Gilead Sciences, and participated on a data safety monitoring board or advisory board for MSD and Pfizer (all paid to the institution). All other authors declare no competing interests.
Figures

References
-
- World Health Organization. Update on the transition to dolutegravir-based antiretroviral therapy: report of a WHO meeting, 29–30 March 2022. 2022. https://www.who.int/publications/i/item/9789240053335.
-
- Bansi-Matharu L, Phillips A, Oprea C, et al. Contemporary antiretrovirals and body-mass index: a prospective study of the RESPOND cohort consortium. Lancet HIV 2021; 8: e711–22. - PubMed
-
- Antonello VS, Carlos Ferreira Antonello I, Grossmann TK, Tovo CV, Brasil Dal Pupo B, De Quadros Winckler L. Hypertension - An emerging cardiovascular risk factor in HIV infection. Journal of the American Society of Hypertension 2015; 9: 403–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

