Dyrk1a is required for craniofacial development in Xenopus laevis
- PMID: 38621649
- PMCID: PMC12024765
- DOI: 10.1016/j.ydbio.2024.04.004
Dyrk1a is required for craniofacial development in Xenopus laevis
Abstract
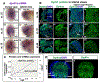
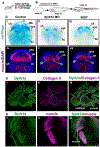
Loss of function variations in the dual specificity tyrosine-phosphorylation-regulated kinase 1 A (DYRK1A) gene are associated with craniofacial malformations in humans. Here we characterized the effects of deficient DYRK1A in craniofacial development using a developmental model, Xenopus laevis. Dyrk1a mRNA and protein were expressed throughout the developing head and both were enriched in the branchial arches which contribute to the face and jaw. Consistently, reduced Dyrk1a function, using dyrk1a morpholinos and pharmacological inhibitors, resulted in orofacial malformations including hypotelorism, altered mouth shape, slanted eyes, and narrower face accompanied by smaller jaw cartilage and muscle. Inhibition of Dyrk1a function resulted in misexpression of key craniofacial regulators including transcription factors and members of the retinoic acid signaling pathway. Two such regulators, sox9 and pax3 are required for neural crest development and their decreased expression corresponds with smaller neural crest domains within the branchial arches. Finally, we determined that the smaller size of the faces, jaw elements and neural crest domains in embryos deficient in Dyrk1a could be explained by increased cell death and decreased proliferation. This study is the first to provide insight into why craniofacial birth defects might arise in humans with variants of DYRK1A.
Keywords: Craniofacial; DYRK1A; Xenopus laevis.
Published by Elsevier Inc.
Figures


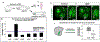

Update of
-
Dyrk1a is required for craniofacial development in Xenopus laevis.bioRxiv [Preprint]. 2024 Jan 14:2024.01.13.575394. doi: 10.1101/2024.01.13.575394. bioRxiv. 2024. Update in: Dev Biol. 2024 Jul;511:63-75. doi: 10.1016/j.ydbio.2024.04.004. PMID: 38260562 Free PMC article. Updated. Preprint.
References
-
- Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, Neilson JR, Chen L, Heit JJ, Kim SK, Yamasaki N, Miyakawa T, Francke U, Graef IA, Crabtree GR, 2006. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 441, 595–600. - PubMed
-
- Asher JH Jr., Sommer A, Morell R, Friedman TB, 1996. Missense mutation in the paired domain of PAX3 causes craniofacial-deafness-hand syndrome. Hum. Mutat 7, 30–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

