Late-Stage Saturation of Drug Molecules
- PMID: 38621677
- PMCID: PMC11066876
- DOI: 10.1021/jacs.4c00807
Late-Stage Saturation of Drug Molecules
Abstract
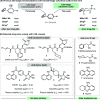
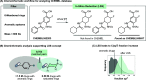
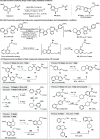
The available methods of chemical synthesis have arguably contributed to the prevalence of aromatic rings, such as benzene, toluene, xylene, or pyridine, in modern pharmaceuticals. Many such sp2-carbon-rich fragments are now easy to synthesize using high-quality cross-coupling reactions that click together an ever-expanding menu of commercially available building blocks, but the products are flat and lipophilic, decreasing their odds of becoming marketed drugs. Converting flat aromatic molecules into saturated analogues with a higher fraction of sp3 carbons could improve their medicinal properties and facilitate the invention of safe, efficacious, metabolically stable, and soluble medicines. In this study, we show that aromatic and heteroaromatic drugs can be readily saturated under exceptionally mild rhodium-catalyzed hydrogenation, acid-mediated reduction, or photocatalyzed-hydrogenation conditions, converting sp2 carbon atoms into sp3 carbon atoms and leading to saturated molecules with improved medicinal properties. These methods are productive in diverse pockets of chemical space, producing complex saturated pharmaceuticals bearing a variety of functional groups and three-dimensional architectures. The rhodium-catalyzed method tolerates traces of dimethyl sulfoxide (DMSO) or water, meaning that pharmaceutical compound collections, which are typically stored in wet DMSO, can finally be reformatted for use as substrates for chemical synthesis. This latter application is demonstrated through the late-stage saturation (LSS) of 768 complex and densely functionalized small-molecule drugs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

