Rituximab or cyclosporine A for the treatment of membranous nephropathy: economic evaluation of the MENTOR trial
- PMID: 38621719
- PMCID: PMC11596091
- DOI: 10.1093/ndt/gfae084
Rituximab or cyclosporine A for the treatment of membranous nephropathy: economic evaluation of the MENTOR trial
Abstract
Background and hypothesis: The MENTOR trial (MEmbranous Nephropathy Trial Of Rituximab) showed that rituximab was noninferior to cyclosporine in inducing complete or partial remission of proteinuria and was superior in maintaining proteinuria remission. However, the cost of rituximab may prohibit first-line use for some patients and health-care payers.
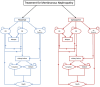
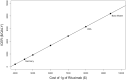
Methods: A Markov model was used to determine the incremental cost-effectiveness ratio (ICER) of rituximab compared with cyclosporine for the treatment membranous nephropathy from the perspective of a health-care payer with a lifetime time horizon. The model was informed by data from the MENTOR trial where possible; additional parameters including cost and utility inputs were obtained from the literature. Sensitivity analyses were performed to evaluate the impact of reduced-cost biosimilar rituximab.
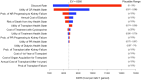
Results: Rituximab for the treatment of membranous nephropathy was cost effective (assuming a willingness-to-pay threshold of $50 000 per quality-adjusted life year (QALY) gained; in $US 2021) compared with cyclosporine, with an ICER of $8373/QALY over a lifetime time horizon. The incremental cost of rituximab therapy was $28 007 with an additional 3.34 QALYs compared with cyclosporine. Lower cost of rituximab biosimilars resulted in a more favorable ICER, and in some cases resulted in rituximab being dominant (lower cost and great benefit) compared to cyclosporine.
Conclusions: Despite the greater cost of rituximab, it may be a cost-effective option for the treatment of membranous nephropathy when compared with cyclosporine. The cost-effectiveness of rituximab is further improved with the use of less expensive biosimilars.
Keywords: cost-effectiveness; cyclosporine; economic evaluation; membranous nephropathy; rituximab.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
Sources of support: M.K. receives financial support from the Michael Smith health Research BC Health Professional Investigator Award. S.K. is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta. S.K. is Director of the Real World Evidence Consortium, and Alberta Drug and Therapeutic Evaluation Consortium (Universities of Alberta, Calgary, and Institute of Health Economics); these entities receive funding from decision-makers and industry to conduct research. All research funding is made to the academic institution; investigator retains full rights of academic freedom and right to publish. This relationship is not related to the current work.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

