Tetracyclines enhance antitumor T-cell immunity via the Zap70 signaling pathway
- PMID: 38621815
- PMCID: PMC11328671
- DOI: 10.1136/jitc-2023-008334
Tetracyclines enhance antitumor T-cell immunity via the Zap70 signaling pathway
Abstract
Background: Cancer immunotherapy including immune checkpoint inhibitors is only effective for a limited population of patients with cancer. Therefore, the development of novel cancer immunotherapy is anticipated. In preliminary studies, we demonstrated that tetracyclines enhanced T-cell responses. Therefore, we herein investigated the efficacy of tetracyclines on antitumor T-cell responses by human peripheral T cells, murine models, and the lung tumor tissues of patients with non-small cell lung cancer (NSCLC), with a focus on signaling pathways in T cells.
Methods: The cytotoxicity of peripheral and lung tumor-infiltrated human T cells against tumor cells was assessed by using bispecific T-cell engager (BiTE) technology (BiTE-assay system). The effects of tetracyclines on T cells in the peripheral blood of healthy donors and the tumor tissues of patients with NSCLC were examined using the BiTE-assay system in comparison with anti-programmed cell death-1 (PD-1) antibody, nivolumab. T-cell signaling molecules were analyzed by flow cytometry, ELISA, and qRT-PCR. To investigate the in vivo antitumor effects of tetracyclines, tetracyclines were administered orally to BALB/c mice engrafted with murine tumor cell lines, either in the presence or absence of anti-mouse CD8 inhibitors.
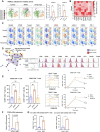
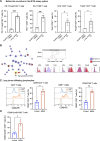
Results: The results obtained revealed that tetracyclines enhanced antitumor T-cell cytotoxicity with the upregulation of granzyme B and increased secretion of interferon-γ in human peripheral T cells and the lung tumor tissues of patients with NSCLC. The analysis of T-cell signaling showed that CD69 in both CD4+ and CD8+ T cells was upregulated by minocycline. Downstream of T-cell receptor signaling, Zap70 phosphorylation and Nur77 were also upregulated by minocycline in the early phase after T-cell activation. These changes were not observed in T cells treated with anti-PD-1 antibodies under the same conditions. The administration of tetracyclines exhibited antitumor efficacy with the upregulation of CD69 and increases in tumor antigen-specific T cells in murine tumor models. These changes were canceled by the administration of anti-mouse CD8 inhibitors.
Conclusions: In conclusion, tetracyclines enhanced antitumor T-cell immunity via Zap70 signaling. These results will contribute to the development of novel cancer immunotherapy.
Keywords: Drug Evaluation, Preclinical; Non-Small Cell Lung Cancer; T-Lymphocytes.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: There are no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
