Recurrent RhoGAP gene fusion CLDN18-ARHGAP26 promotes RHOA activation and focal adhesion kinase and YAP-TEAD signalling in diffuse gastric cancer
- PMID: 38621923
- PMCID: PMC11287566
- DOI: 10.1136/gutjnl-2023-329686
Recurrent RhoGAP gene fusion CLDN18-ARHGAP26 promotes RHOA activation and focal adhesion kinase and YAP-TEAD signalling in diffuse gastric cancer
Abstract
Objective: Genomic studies of gastric cancer have identified highly recurrent genomic alterations impacting RHO signalling, especially in the diffuse gastric cancer (DGC) histological subtype. Among these alterations are interchromosomal translations leading to the fusion of the adhesion protein CLDN18 and RHO regulator ARHGAP26. It remains unclear how these fusion constructs impact the activity of the RHO pathway and what is their broader impact on gastric cancer development. Herein, we developed a model to allow us to study the function of this fusion protein in the pathogenesis of DGC and to identify potential therapeutic targets for DGC tumours with these alterations.
Design: We built a transgenic mouse model with LSL-CLDN18-ARHGAP26 fusion engineered into the Col1A1 locus where its expression can be induced by Cre recombinase. Using organoids generated from this model, we evaluated its oncogenic activity and the biochemical effects of the fusion protein on the RHOA pathway and its downstream cell biological effects in the pathogenesis of DGC.
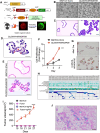
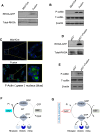
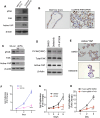
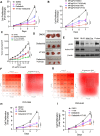
Results: We demonstrated that induction of CLDN18-ARHGAP26 expression in gastric organoids induced the formation of signet ring cells, characteristic features of DGC and was able to cooperatively transform gastric cells when combined with the loss of the tumour suppressor geneTrp53. CLDN18-ARHGAP26 promotes the activation of RHOA and downstream effector signalling. Molecularly, the fusion promotes activation of the focal adhesion kinase (FAK) and induction of the YAP pathway. A combination of FAK and YAP/TEAD inhibition can significantly block tumour growth.
Conclusion: These results indicate that the CLDN18-ARHGAP26 fusion is a gain-of-function DGC oncogene that leads to activation of RHOA and activation of FAK and YAP signalling. These results argue for further evaluation of emerging FAK and YAP-TEAD inhibitors for these deadly cancers.
Keywords: gastric cancer; molecular oncology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AJB has received funding from Merck, Novartis, Bayer and Repare, is the advisor to Earli and HelixNano and is now employed by the Novartis Institutes of Biomedical Research. AJB and HZ are co-founders and equity holders in Signet Therapeutics. CJD is an advisory board member for Deciphera Pharmaceuticals, Mirati Therapeutics, Reactive Biosciences, Revolution Medicines and SHY Therapeutics; has received research funding support from Boragen, Deciphera Pharmaceuticals, Mirati Therapeutics, Revolution Medicines and SpringWorks Therapeutics; and has consulted for Day One Biotherapeutics, Eli Lilly, Jazz Therapeutics, Ribometrix, Sanofi and Turning Point Therapeutics. All other authors do not report conflicts.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
