The effect of white grub (Maladera Verticalis) larvae feeding on rhizosphere microbial characterization of aerobic rice (Oryza sativa L.) in Puer City, Yunnan Province, China
- PMID: 38622504
- PMCID: PMC11017655
- DOI: 10.1186/s12866-024-03265-w
The effect of white grub (Maladera Verticalis) larvae feeding on rhizosphere microbial characterization of aerobic rice (Oryza sativa L.) in Puer City, Yunnan Province, China
Abstract
Background: Rhizosphere microorganisms are vital in plants' growth and development and these beneficial microbes are recruited to the root-zone soil when experiencing various environmental stresses. However, the effect of white grub (Maladera verticalis) larvae feeding on the structure and function of rhizosphere microbial communities of aerobic rice (Oryza sativa L.) is unclear.
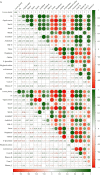
Results: In this study, we compared physicochemical properties, enzyme activities, and microbial communities using 18 samples under healthy and M. verticalis larvae-feeding aerobic rice rhizosphere soils at the Yunnan of China. 16 S rRNA and ITS amplicons were sequenced using Illumina high throughput sequencing. M. verticalis larvae feeding on aerobic rice can influence rhizosphere soil physicochemical properties and enzyme activities, which also change rhizosphere microbial communities. The healthy and M. verticalis larvae-feeding aerobic rice rhizosphere soil microorganisms had distinct genus signatures, such as possible_genus_04 and Knoellia genera in healthy aerobic rice rhizosphere soils and norank_f__SC - I-84 and norank_f__Roseiflexaceae genera in M. verticalis larvae-feeding aerobic rice rhizosphere soils. The pathway of the metabolism of terpenoids and polyketides and carbohydrate metabolism in rhizosphere bacteria were significantly decreased after M. verticalis larvae feeding. Fungal parasite-wood saprotroph and fungal parasites were significantly decreased after M. verticalis larvae feeding, and plant pathogen-wood saprotroph and animal pathogen-undefined saprotroph were increased after larvae feeding. Additionally, the relative abundance of Bradyrhizobium and Talaromyces genera gradually increased with the elevation of the larvae density. Bacterial and fungal communities significantly correlated with soil physicochemical properties and enzyme activities, respectively.
Conclusions: Based on the results we provide new insight for understanding the adaptation of aerobic rice to M. verticalis larvae feeding via regulating the rhizosphere environment, which would allow us to facilitate translation to more effective measures.
Keywords: Maladera Verticalis; Oryza sativa; Aerobic rice; Enzyme activities; Physicochemical properties; Rhizosphere microorganisms; White grub.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Barker R, Dawe D, Tuong TP, Bhuiyan SI, Guerra L. The outlook for water resources in the year 2020: Challenges for research on water management in rice production. Proceedings of 19th Session of the International Rice Commission 1999:96–109.
-
- Bouman B, Lampayan R, Tuong TP. Water management in irrigated rice. Coping with water scarcity; 2007.
-
- Hugar S, Naik M, Munishamappa M. Comparative Biology of Yellow Stem Borer, Scirpophaga incertulas Walker in Aerobic and Transplanted Rice.
-
- Kr R, Nath, Deka S. Insect pests of citrus and their management ARITCLE INFO a CASE STUDY. 2020, 12:188–96.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

