Interferon-γ-induced GBP1 is an inhibitor of human papillomavirus 18
- PMID: 38622605
- PMCID: PMC11017553
- DOI: 10.1186/s12905-024-03057-4
Interferon-γ-induced GBP1 is an inhibitor of human papillomavirus 18
Abstract
Background: Human papillomavirus (HPV) infection is an important factor leading to cervical cell abnormalities. 90% of cervical cancers are closely associated with persistent infection of high-risk HPV, with the highest correlation with HPV16 and 18. Currently available vaccines and antivirals have limited effectiveness and coverage. Guanylate binding protein 1 (GBP1) was induced by interferon gamma and involved in many important cellular processes such as clearance of various microbial pathogens. However, whether GBP1 can inhibit human papillomavirus infection is unclear.
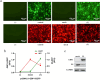
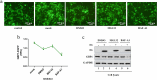
Results: In this study, we found that GBP1 can effectively degrade HPV18 E6, possibly through its GTPase activity or other pathways, and E6 protein degrades GBP1 through the ubiquitin-proteasome pathway to achieve immune escape.
Conclusion: Therefore, GBP1 is an effector of IFN-γ anti-HPV activity. Our findings provided new insights into the treatment of HPV 18 infections.
Keywords: Guanylate-binding protein 1; Human papillomavirus 18; Interferon gamma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Messenger ribonucleic acid encoding interferon-inducible guanylate binding protein 1 is induced in human endometrium within the putative window of implantation.J Clin Endocrinol Metab. 2001 Jun;86(6):2420-7. doi: 10.1210/jcem.86.6.7534. J Clin Endocrinol Metab. 2001. PMID: 11397834
-
IFN-gamma produced by human papilloma virus-18 E6-specific CD4+ T cells predicts the clinical outcome after surgery in patients with high-grade cervical lesions.J Immunol. 2007 Nov 15;179(10):7176-83. doi: 10.4049/jimmunol.179.10.7176. J Immunol. 2007. PMID: 17982110
-
Human Papillomavirus (HPV) 16/18 E6 Oncoprotein Expression in Infections with Single and Multiple Genotypes.Cancer Prev Res (Phila). 2019 Feb;12(2):95-102. doi: 10.1158/1940-6207.CAPR-18-0343. Epub 2019 Jan 3. Cancer Prev Res (Phila). 2019. PMID: 30606718
-
Guanylate-Binding Protein 1: An Emerging Target in Inflammation and Cancer.Front Immunol. 2020 Jan 24;10:3139. doi: 10.3389/fimmu.2019.03139. eCollection 2019. Front Immunol. 2020. PMID: 32117203 Free PMC article. Review.
-
[Recent progress in interferon induced protein GBP1 research].Bing Du Xue Bao. 2014 Jul;30(4):456-62. Bing Du Xue Bao. 2014. PMID: 25272603 Review. Chinese.
Cited by
-
Advances in the Prevention of Cervical Cancer by Anti-Human Papillomavirus Agents.Cancer Med. 2025 Apr;14(7):e70847. doi: 10.1002/cam4.70847. Cancer Med. 2025. PMID: 40189844 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

