Interferon-α stimulates DExH-box helicase 58 to prevent hepatocyte ferroptosis
- PMID: 38622688
- PMCID: PMC11017495
- DOI: 10.1186/s40779-024-00524-9
Interferon-α stimulates DExH-box helicase 58 to prevent hepatocyte ferroptosis
Abstract
Background: Liver ischemia/reperfusion (I/R) injury is usually caused by hepatic inflow occlusion during liver surgery, and is frequently observed during war wounds and trauma. Hepatocyte ferroptosis plays a critical role in liver I/R injury, however, it remains unclear whether this process is controlled or regulated by members of the DEAD/DExH-box helicase (DDX/DHX) family.
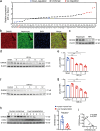
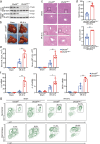
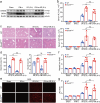
Methods: The expression of DDX/DHX family members during liver I/R injury was screened using transcriptome analysis. Hepatocyte-specific Dhx58 knockout mice were constructed, and a partial liver I/R operation was performed. Single-cell RNA sequencing (scRNA-seq) in the liver post I/R suggested enhanced ferroptosis by Dhx58hep-/-. The mRNAs and proteins associated with DExH-box helicase 58 (DHX58) were screened using RNA immunoprecipitation-sequencing (RIP-seq) and IP-mass spectrometry (IP-MS).
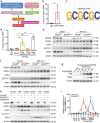
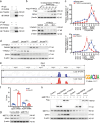
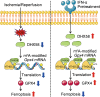
Results: Excessive production of reactive oxygen species (ROS) decreased the expression of the IFN-stimulated gene Dhx58 in hepatocytes and promoted hepatic ferroptosis, while treatment using IFN-α increased DHX58 expression and prevented ferroptosis during liver I/R injury. Mechanistically, DHX58 with RNA-binding activity constitutively associates with the mRNA of glutathione peroxidase 4 (GPX4), a central ferroptosis suppressor, and recruits the m6A reader YT521-B homology domain containing 2 (YTHDC2) to promote the translation of Gpx4 mRNA in an m6A-dependent manner, thus enhancing GPX4 protein levels and preventing hepatic ferroptosis.
Conclusions: This study provides mechanistic evidence that IFN-α stimulates DHX58 to promote the translation of m6A-modified Gpx4 mRNA, suggesting the potential clinical application of IFN-α in the prevention of hepatic ferroptosis during liver I/R injury.
Keywords: DExH-box helicase 58 (DHX58); Glutathione peroxidase 4 (GPX4); Ischemia/reperfusion (I/R); YT521-B homology domain containing 2 (YTHDC2); m6A modification.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

