The role of the brain renin-angiotensin system in Parkinson´s disease
- PMID: 38622720
- PMCID: PMC11017622
- DOI: 10.1186/s40035-024-00410-3
The role of the brain renin-angiotensin system in Parkinson´s disease
Abstract
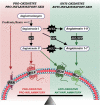
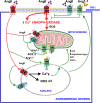
The renin-angiotensin system (RAS) was classically considered a circulating hormonal system that regulates blood pressure. However, different tissues and organs, including the brain, have a local paracrine RAS. Mutual regulation between the dopaminergic system and RAS has been observed in several tissues. Dysregulation of these interactions leads to renal and cardiovascular diseases, as well as progression of dopaminergic neuron degeneration in a major brain center of dopamine/angiotensin interaction such as the nigrostriatal system. A decrease in the dopaminergic function induces upregulation of the angiotensin type-1 (AT1) receptor activity, leading to recovery of dopamine levels. However, AT1 receptor overactivity in dopaminergic neurons and microglial cells upregulates the cellular NADPH-oxidase-superoxide axis and Ca2+ release, which mediate several key events in oxidative stress, neuroinflammation, and α-synuclein aggregation, involved in Parkinson's disease (PD) pathogenesis. An intraneuronal antioxidative/anti-inflammatory RAS counteracts the effects of the pro-oxidative AT1 receptor overactivity. Consistent with this, an imbalance in RAS activity towards the pro-oxidative/pro-inflammatory AT1 receptor axis has been observed in the substantia nigra and striatum of several animal models of high vulnerability to dopaminergic degeneration. Interestingly, autoantibodies against angiotensin-converting enzyme 2 and AT1 receptors are increased in PD models and PD patients and contribute to blood-brain barrier (BBB) dysregulation and nigrostriatal pro-inflammatory RAS upregulation. Therapeutic strategies addressed to the modulation of brain RAS, by AT1 receptor blockers (ARBs) and/or activation of the antioxidative axis (AT2, Mas receptors), may be neuroprotective for individuals with a high risk of developing PD or in prodromal stages of PD to reduce progression of the disease.
Keywords: Angiotensin; Dopamine; NADPH-oxidase; Neurodegeneration; Neuroinflammation; Neuroprotection; Oxidative stress; Parkinson.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- Tigerstedt R, Bergman PQ. Niere und kreislauf. Skand Arch Physiol. 1898;8:223–71. doi: 10.1111/j.1748-1716.1898.tb00272.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

