Increased susceptibility to azithromycin of Pseudomonas aeruginosa biofilms using RPMI 1640 testing media
- PMID: 38622982
- PMCID: PMC11582341
- DOI: 10.1111/apm.13413
Increased susceptibility to azithromycin of Pseudomonas aeruginosa biofilms using RPMI 1640 testing media
Abstract
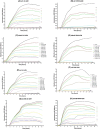
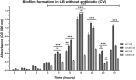
Azithromycin (AZM) is efficient for treatment of chronic Pseudomonas aeruginosa biofilm lung infections, despite of resistance in conventional susceptibility testing. It has been shown that planktonic P. aeruginosa are more susceptible to AZM when tested in RPMI 1640 medium. The aim of the study was to test the susceptibility to AZM of P. aeruginosa biofilms in LB vs RPMI 1640 media. We investigated the effect of AZM on planktonic and biofilms of (WT) P. aeruginosa (PAO1), the hypermutable (ΔmutS) and the antibiotic-resistant phenotype(ΔnfxB) mutants. The effect of AZM on young and mature biofilms was investigated in the modified Calgary Biofilm Device by estimation of the minimal biofilm inhibitory concentration (MBIC). The AZM MBIC90 in LB/RPMI1640 on young biofilms treated for 24 h was 16/4 μg/mL for PAO1, 32/8 μg/mL for ΔmutS, and 256/16 μg/mL for ΔnfxB, while in mature biofilms was 256/2 μg/mL for PAO1 and ΔmutS and 16/1 μg/mL for ΔnfxB. The effect of AZM was improved when the treatment was prolonged to 72 h, supporting the intracellular accumulation of AZM. An increased susceptibility of P. aeruginosa biofilms to AZM was observed in RPMI 1640 than in LB medium. Our results might improve susceptibility testing and dosing of AZM for treatment of biofilm infections.
Keywords: Pseudomonas aeruginosa; RPMI‐1640; azithromycin; biofilm.
© 2024 The Authors. APMIS published by John Wiley & Sons Ltd on behalf of Scandinavian Societies for Pathology, Medical Microbiology and Immunology.
Figures


References
-
- Saiman L, Marshall BC, Mayer‐Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. - PubMed
-
- Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EHJ, Koppers RJH, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013;309:1251–1259. - PubMed
-
- Jaffe A, Bush A. Anti‐inflammatory effects of macrolides in lung disease. Pediatr Pulmonol. 2001;31:464–473. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

