Polyester Nanoparticles with Controlled Topography for Peroral Drug Delivery Using Insulin as a Model Protein
- PMID: 38622996
- PMCID: PMC11145941
- DOI: 10.1021/acsnano.4c01027
Polyester Nanoparticles with Controlled Topography for Peroral Drug Delivery Using Insulin as a Model Protein
Abstract
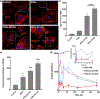
Receptor-mediated polyester drug delivery systems have tremendous potential for improving the clinical performance of existing pharmaceutical drugs. Despite significant progress made in this area, it remains unclear how and to what extent the polyester nanoparticle surface topography would affect the in vitro, ex vivo and in vivo performance of a drug, and if there exists a correlation between in vitro and in vivo, as well as healthy versus pathophysiological states. Herein, we report a systematic investigation of the interactions between ligands and receptors as a function of the linker length, two-carbon (2C) versus four-carbon (4C). The in vitro, ex vivo and in vivo in healthy models validate the hypothesis that 4C has better reach and binding to the receptors. The results indicate that 4C offered better performance over 2C in vivo in improving the oral bioavailability of insulin (INS) by 1.1-fold (3.5-fold compared to unfunctionalized nanoparticles) in a healthy rat model. Similar observations were made in pathophysiological models; however, the effects were less prominent compared to those in healthy models. Throughout, ligand decorated nanoparticles outperformed unfunctionalized nanoparticles. Finally, a semimechanistic pharmacokinetic and pharmacodynamic (PKPD) model was developed using the experimental data sets to quantitatively evaluate the effect of P2Ns-GA on oral bioavailability and efficacy of insulin. The study presents a sophisticated oral delivery system for INS or hydrophilic therapeutic cargo, highlighting the significant impact on bioavailability that minor adjustments to the surface chemistry can have.
Keywords: nanobiointerface; nanotopography; oral insulin delivery; polyester nanoparticles; receptor-mediated transcytosis.
Figures



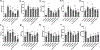

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

