Necl-1/CADM3 regulates cone synapse formation in the mouse retina
- PMID: 38623325
- PMCID: PMC11016759
- DOI: 10.1016/j.isci.2024.109577
Necl-1/CADM3 regulates cone synapse formation in the mouse retina
Abstract
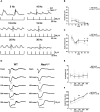
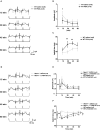
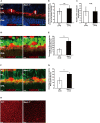
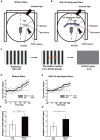
In vertebrates, retinal neural circuitry for visual perception is organized in specific layers. The outer plexiform layer is the first synaptic region in the visual pathway, where photoreceptor synaptic terminals connect with bipolar and horizontal cell processes. However, molecular mechanisms underlying cone synapse formation to mediate OFF pathways remain unknown. This study reveals that Necl-1/CADM3 is localized at S- and S/M-opsin-containing cones and dendrites of type 4 OFF cone bipolar cells (CBCs). In Necl-1-/- mouse retina, synapses between cones and type 4 OFF CBCs were dislocated, horizontal cell distribution became abnormal, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors were dislocated. Necl-1-/- mice exhibited aberrant short-wavelength-light-elicited signal transmission from cones to OFF CBCs, which was rescued by AMPA receptor potentiator. Additionally, Necl-1-/- mice showed impaired optokinetic responses. These findings suggest that Necl-1 regulates cone synapse formation to mediate OFF cone pathways elicited by short-wavelength light in mouse retina.
Keywords: Biological sciences; Molecular neuroscience; Neuroscience;.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

