Combination of T cell-redirecting strategies with a bispecific antibody blocking TGF-β and PD-L1 enhances antitumor responses
- PMID: 38623463
- PMCID: PMC11018002
- DOI: 10.1080/2162402X.2024.2338558
Combination of T cell-redirecting strategies with a bispecific antibody blocking TGF-β and PD-L1 enhances antitumor responses
Abstract
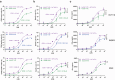
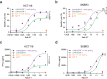
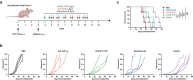
T cell-based immunotherapies for solid tumors have not achieved the clinical success observed in hematological malignancies, partially due to the immunosuppressive effect promoted by the tumor microenvironment, where PD-L1 and TGF-β play a pivotal role. However, durable responses to immune checkpoint inhibitors remain limited to a minority of patients, while TGF-β inhibitors have not reached the market yet. Here, we describe a bispecific antibody for dual blockade of PD-L1 and TFG-β, termed AxF (scFv)2, under the premise that combination with T cell redirecting strategies would improve clinical benefit. The AxF (scFv)2 antibody was well expressed in mammalian and yeast cells, bound both targets and inhibited dose-dependently the corresponding signaling pathways in luminescence-based cellular reporter systems. Moreover, combined treatment with trispecific T-cell engagers (TriTE) or CAR-T cells significantly boosted T cell activation status and cytotoxic response in breast, lung and colorectal (CRC) cancer models. Importantly, the combination of an EpCAMxCD3×EGFR TriTE with the AxF (scFv)2 delayed CRC tumor growth in vivo and significantly enhanced survival compared to monotherapy with the trispecific antibody. In summary, we demonstrated the feasibility of concomitant blockade of PD-L1 and TGF-β by a single molecule, as well as its therapeutic potential in combination with different T cell redirecting agents to overcome tumor microenvironment-mediated immunosuppression.
Keywords: Bispecific antibody; CAR-T cell; T-cell engager; cancer immunotherapy; colorectal cancer; combination therapy; trispecific antibody.
© 2024 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
