Early diagnosis and treatment by newborn screening (NBS) or family history is associated with improved visual outcomes for long-chain 3-hydroxyacylCoA dehydrogenase deficiency (LCHADD) chorioretinopathy
- PMID: 38623632
- PMCID: PMC11251862
- DOI: 10.1002/jimd.12738
Early diagnosis and treatment by newborn screening (NBS) or family history is associated with improved visual outcomes for long-chain 3-hydroxyacylCoA dehydrogenase deficiency (LCHADD) chorioretinopathy
Abstract
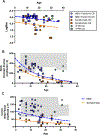
Long chain 3-hydroxyacyl-CoA dehydrogenase (LCHADD) is the only fatty acid oxidation disorder to develop a progressive chorioretinopathy resulting in vision loss; newborn screening (NBS) for this disorder began in the United States around 2004. We compared visual outcomes among 40 participants with LCHADD or trifunctional protein deficiency diagnosed symptomatically to those who were diagnosed via NBS or a family history. Participants completed ophthalmologic testing including measures of visual acuity, electroretinograms (ERG), fundal imaging, contrast sensitivity, and visual fields. Records were reviewed to document medical and treatment history. Twelve participants presented symptomatically with hypoglycemia, failure to thrive, liver dysfunction, cardiac arrest, or rhabdomyolysis. Twenty eight were diagnosed by NBS or due to a family history of LCHADD. Participants diagnosed symptomatically were older but had similar percent males and genotypes as those diagnosed by NBS. Treatment consisted of fasting avoidance, dietary long-chain fat restriction, MCT, C7, and/or carnitine supplementation. Visual acuity, rod- and cone-driven amplitudes on ERG, contrast sensitivity scores, and visual fields were all significantly worse among participants diagnosed symptomatically compared to NBS. In mixed-effects models, both age and presentation (symptomatic vs. NBS) were significant independent factors associated with visual outcomes. This suggests that visual outcomes were improved by NBS, but there was still lower visual function with advancing age in both groups. Early diagnosis and treatment by NBS is associated with improved visual outcomes and retinal function compared to participants who presented symptomatically. Despite the impact of early intervention, chorioretinopathy was greater with advancing age, highlighting the need for novel treatments.
Keywords: chorioretinopathy; long‐chain 3‐hydroxyacylCoA dehydrogenase deficiency; long‐chain fatty acid oxidation disorder; newborn screening.
© 2024 SSIEM.
Conflict of interest statement
Competing Interest Statement:
This is a natural history study and no treatments were investigated. Authors have the following competing interests related to treatment and management of fatty acid oxidation disorders in general or to the development of retinal therapies in other unrelated disorders.
Dongseok Choi, Ashley Gregor, Nida Wongchaisuwat, Danielle Black, Hannah L Scanga and Georgianne Arnold have no competing interest.
Melanie B Gillingham has received speaker honorium from Ultragenyx Pharmaceutical Inc., Vitaflow, and Nutricia, and received research grant/funds from Nestle Bioscience and Reneo Pharmaceutical.
Ken K Nischal had received research funding from Ophtec, ORA, Essilor, consulting fees from Graybug Gene therapies, Krystal Inc, and honoraria from Santen.
Jose-Alain Sahal has equity in the following companies: Gensight, Sparing Vision, Avista, Tenpoint, Prophesee, Chronolife, Tilak Healthcare, SharpEye, Cilensee, and Vegavect. He has received research funding from Gensight, Sparing Vision and Meira, is a consultant for Avista Therapeutics and Tenpoint, and serves on the scientific advisory boards for the Gilbert foundation, the Foundation for Fighting Blindness, Institute of Ophthalmology Basel, and the Senses Institute Lausanne.
Jerry Vockley has received research funding from Ultragenyx Pharmaceutical Inc, Reneo Pharmaceutical and Nestle Bioscience.
Cary O Harding has received research funding from Reneo Pharmaceutical and Nestle Bioscience
Mark E Pennesi has received consulting fees from 4D Molecular Therapeutics, Adverum, Arrowhead Pharmaceuticals, AGTC, Aldebraran, Ascidian, Atsena, Astellas, BlueRock-Opsis, Coave, ClarisBio, Dompe, Editas, Edigene, Endogena, FFB, Ingel Therapeutics J-Cyte, Janssen, KalaTherapeutics, Kiora, Nacuity Pharmaceuticals, Ocugen, Ora, ProQR, Prime Editing, PTC Therapeutics, PYC Therapeutics, Ray Therapeutics, Rejuvitas, RestoreVision, RegenexBio, Sparing Vision, SpliceBio, Spotlight Therapeutics, Thea and Theranexus. He has received clinical trial support from AGTC, Biogen, Editas, FFB, ProQR, Reneuron. He has received fees as part of the Data Safety Montitoring Board (DSMB) for Akous, Gensight. He has equity in the following companies: Aldebaran, Atsena, Endogena, EnterX, Ingel Therapeutics, Kiora, Nacuity Pharmaceuticals, Ocugen, and ZipBio.
Figures
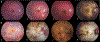



References
-
- Vockley J, Bennett MJ, Gillingham MB. Mitochondiral Fatty Acid Oxisation Disorders. In: Mitchell G, Gibson KM, Ballabio A, et al. , eds. The Online Metabolic and Molecular Bases of Inherited Disease. The McGraw-Hill Companies, Inc.; 2017:chap 101.
-
- Ijlst L, Ruiter JP, Hoovers JM, Jakobs ME, Wanders RJ. Common missense mutation G1528C in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Characterization and expression of the mutant protein, mutation analysis on genomic DNA and chromosomal localization of the mitochondrial trifunctional protein alpha subunit gene. J Clin Invest. 1996;98(4):1028–33. - PMC - PubMed
-
- Ijlst L, Wanders RJ, Ushikubo S, Kamijo T, Hashimoto T. Molecular basis of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of the major disease-causing mutation in the alpha-subunit of the mitochondrial trifunctional protein. Biochimica et Biophysica Acta. 1994;1215(3):347–350. - PubMed
-
- Boutron A, Acquaviva C, Vianey-Saban C, et al. Comprehensive cDNA study and quantitative analysis of mutant HADHA and HADHB transcripts in a French cohort of 52 patients with mitochondrial trifunctional protein deficiency. Mol Genet Metab. Aug 2011;103(4):341–8. doi: 10.1016/j.ymgme.2011.04.006 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

