Efficacy and safety of novel immune checkpoint inhibitor-based combinations versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer: A network meta-analysis
- PMID: 38623838
- PMCID: PMC11128374
- DOI: 10.1111/1759-7714.15310
Efficacy and safety of novel immune checkpoint inhibitor-based combinations versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer: A network meta-analysis
Abstract
Background: Patients with extensive-stage small cell lung cancer (ES-SCLC) have an exceptionally poor prognosis and immune checkpoint inhibitors (ICIs) combined with etoposide-platinum is recommended as standard first-line therapy. However, which combination pattern is the best still remains unknown. This network meta-analysis was performed to compare the efficacy and safety of currently available patterns including an antiangiogenic agent containing regimen and probed into the most appropriate therapy for patients.
Methods: Hazard ratios (HRs) and odds ratios (ORs) were generated using R software. The outcomes of overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events of grade 3 or higher (grade ≥ 3 adverse events [AEs]) were analyzed.
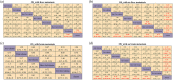
Results: A total of 10 randomized controlled trials (RCTs) involving 5544 patients were included for analysis. Drug combination patterns included adebrelimab, atezolizumab, durvalumab, durvalumab plus tremelimumab, ipilimumab, pembrolizumab, serplulimab, benmelstobart plus anlotinib, tislelizumab, tiragolumab plus atezolizumab and toripalimab in combination with chemotherapy. The novel antiangiogenic agent containing regimen benmelstobart + anlotinib + chemotherapy showed the highest possibility to present the best PFS and OS versus chemotherapy. Compared with ICI plus chemotherapy, it also achieved significantly better PFS and presented a tendency of OS benefit. As for safety and toxicity, patients treated with benmelstobart + anlotinib + chemotherapy and durvalumab + tremelimumab + chemotherapy suffered a higher likelihood of more grade ≥ 3 AEs without unexpected AEs.
Conclusion: PD-1/PD-L1 inhibitors-based combinations are associated with significant improvement in both PFS and OS for treatment-naïve ES-SCLC patients. Benmelstobart plus anlotinib with chemotherapy (CT) yielded better survival benefit versus CT alone or other ICIs + CT with caution for more adverse effects along with the addition of an antiangiogenic agent.
Keywords: angiogenesis; chemotherapy; immunology; network meta‐analysis; small cell lung cancer.
© 2024 The Authors. Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Gazdar AF, Minna JD. Developing new, rational therapies for recalcitrant small cell lung cancer. J Natl Cancer Inst. 2016;108(10):djw119. - PubMed
-
- Paz‐Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): a randomised, controlled, open‐label, phase 3 trial. The Lancet. 2019;394(10212):1929–1939. - PubMed
-
- Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, et al. Clinical insights into small cell lung cancer: tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. 2023;73:620–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

