Longitudinal cerebral perfusion in presymptomatic genetic frontotemporal dementia: GENFI results
- PMID: 38623902
- PMCID: PMC11095434
- DOI: 10.1002/alz.13750
Longitudinal cerebral perfusion in presymptomatic genetic frontotemporal dementia: GENFI results
Abstract
Introduction: Effective longitudinal biomarkers that track disease progression are needed to characterize the presymptomatic phase of genetic frontotemporal dementia (FTD). We investigate the utility of cerebral perfusion as one such biomarker in presymptomatic FTD mutation carriers.
Methods: We investigated longitudinal profiles of cerebral perfusion using arterial spin labeling magnetic resonance imaging in 42 C9orf72, 70 GRN, and 31 MAPT presymptomatic carriers and 158 non-carrier controls. Linear mixed effects models assessed perfusion up to 5 years after baseline assessment.
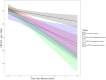
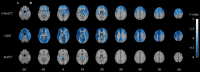
Results: Perfusion decline was evident in all three presymptomatic groups in global gray matter. Each group also featured its own regional pattern of hypoperfusion over time, with the left thalamus common to all groups. Frontal lobe regions featured lower perfusion in those who symptomatically converted versus asymptomatic carriers past their expected age of disease onset.
Discussion: Cerebral perfusion is a potential biomarker for assessing genetic FTD and its genetic subgroups prior to symptom onset.
Highlights: Gray matter perfusion declines in at-risk genetic frontotemporal dementia (FTD). Regional perfusion decline differs between at-risk genetic FTD subgroups . Hypoperfusion in the left thalamus is common across all presymptomatic groups. Converters exhibit greater right frontal hypoperfusion than non-converters past their expected conversion date. Cerebral hypoperfusion is a potential early biomarker of genetic FTD.
Keywords: arterial spin labeling; cerebral perfusion; frontotemporal dementia; presymptomatic biomarker.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M.M. holds additional grants unrelated to this work from the Ontario Brain Institute, Washington University, as well as the Women's Brain Health Initiative and Brain Canada as part of the EU Joint Program for Neurodegenerative Disease Research; has clinical trials contracts with Roche and Alector; has received consulting fees from Ionis, Alector, Biogen Canada, Wave Life Science, Eisai Canada, and Novo Nordisk Canada; received royalties from the Henry Stewart Talks; has received payments from MINT Memory Clinics and ECHO Dementia Series; and holds unpaid Scientific Advisory Board roles with Alzheimer's Society Canada and Parkinson Canada. A.P.S. is a member on the Board of Directors for Parkinson Canada and the Canadian Academy of Health Sciences. B.B. is waiting on a patent on therapeutic intervention in genetic frontotemporal dementia and has received personal fees from UCB, Lilly, AviadoBio, and Denali. C.G. has received payment from Demensdagarna Örebro 2023, Diakonia Ersta sjukhus, and Göteborgsregionen 2023 and is involved as a leader of the Swedish FTD Initiative. D.C. received support from Alzheimer's Research UK (ARUK‐PG2017‐1946), the UCL/UCLH NIHR Biomedical Research Centre, and the UK Dementia Research Institute, which receives its funding from DRI Ltd; and holds a chair position in the Alzheimer's Association Neuroimaging Professional Interest Area. D.T.W. holds an unpaid medical advisory board member position for Hydrocephalus Canada. F.M. holds grants from the Tau Consortium (#A1133749), and the Carlos III Health Institute (PI19/01637). I.S. has participated on the board of Novo Nordisk. J.L. has received support from the DFG German Research Foundation under Germany's Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198); has received personal fees from EISAI and Biogen; has received payments from Abbview, Bayer Vital, Biogen, EISAI, TEVA, Roche, and Zambon; and is on an advisory board for Axon Neuroscience. J.P. holds grants with the Eurostars‐2 joint programme with co‐funding from the European Union Horizon 2020 research and innovation program (ASPIRE E!113701), the Dutch Heart Foundation (2020T049), the EU Joint Program for Neurodegenerative Disease Research, provided by the Netherlands Organisation for Health Research and Development and Alzheimer Nederland DEBBIE (JPND2020‐568‐106), and the Czech Health Research Council (NU23‐08‐00460). J.B.R. has received consulting fees from Astex, Curasen, UCB, WAVE, Prevail, and SVHealth; and is a participant on the board of Asceneuronl, an Associate Director at the Dementias Platform UK, and a Medical Advisor to both Cumulus Neuro and Astronautx. L.L.R. received support from the Guarantors of Brain and Alzheimer's Research UK. M.S. has received payments from UCB, Prevail, Ionis, Orphazyme, Servier, Reata, AviadoBio, GenOrph, Biohaven, Zavra, and Lilly. M.C.T. has received support from NIH and the Weston Brain Foundation; holds positions as a scientific advisor in the Women's Brain Project, Brain Injury Canada, and PSP Canada; and is involved in clinical trials conducted by Janssen, Biogen, Avanex, Green Valley, and Roche. M.F. is listed on a patent related to methods and kits for differential diagnosis of Alzheimer's disease versus frontotemporal dementia using blood biomarkers. J.D.R. participated on advisory boards for Aviado Bio, Arkuda Therapeutics, Prevail Therapeutics, Denali, and Wave Life Sciences. R.S.V. has received additional support from Sage Pharmaceuticals, outside the present study; has received consulting fees from Ionis, AviadoBio, Novo Nordisk, Pfizer, and Lilly; has received payments from Neuraxpharma and Roche Diagnostics; has received travel support from Esteve; and participates on the board of Wave Pharmaceuticals. R.V. is in contract with Alector, Denali, Eli Lilly, J&J, UCB, and Biogen; and participates on the boards of AC Immune, and Novartis. S.E.B. has received contracts from Genentech, Optina, Roche, Eli Lilly, Eisa/Biogen Idec, Novo Nordisk, Lilly Avid, and ICON; has received consulting fees from Roche, Biogen, Novo Nordisk, Eisai, and Eli Lilly; has received payments from Biogen, Roche New England Journal Manuscript, Roche Models of Care Analysis in Canada, and Eisai; and has participated on the boards of the Conference Board of Canada, World Dementia Council, University of Rochester Contribution to the Mission and Scientific Leadership of the Small Vessel VCID Biomarker Validation Consortium, and the National Institute of Neurological Disorders and Stroke. S.D. has been sponsored by Biogen, Novo Nordisk, Janssen, Alnylam, Wave Life Sciences, and Passage Bio; has received consulting fees from Eisai, QuRALIS, AI Therapeutics, and Eli Lilly; has received payments from Eisai; and has participated in the boards of IntelGenX and AVIADO Bio. M.O. reports receiving funding from BMBF – FTLD Consortium, the ALS Association, and EU‐MIRAIDE; has received consulting fees from Biogen, Axon, Roche, and Grifols; has patents with Foundation state Baden‐Wuerttemberg for beta‐Syn as a biomarker for neurodegenerative diseases; and participates on the Biogen ATLAS trail board; is a speaker for the FTLD consortium, is involved in an unpaid role with the German Society for CSF Diagnostics and Neurochemistry, and is involved without pay with the Society for CSF Diagnostics and Neurochemistry. M.P., S.M., N.L., H.J.J.M.M., D.T., M.B., S.B.M., E.R., A.B., J.C.vS., L.C.J., H.S., R.L., P.T., D.G., E.F., S.S., A.dM., C.B., A.G., and B.J.M. report no conflicts. Author disclosures are available in the supporting information.
Figures
References
Publication types
MeSH terms
Grants and funding
- MOP-327387/CIHR/Canada
- PJT-175242/CIHR/Canada
- MOP-327387/CIHR/Canada
- PJT-175242/CIHR/Canada
- Weston Brain Institute
- MR/M023664/1/Medical Research Council UK
- DLR/BMBF 2019- 02248/JPND GENFI-PROX
- Deutsche Forschungsgemeinschaft
- EXC 2145 SyNergy - ID 390857198/Munich Cluster for Systems Neurology
- MR/M008525/1/MRC Clinician Scientist Fellowship
- BRC149/NS/MH/NIHR Rare Disease Translational Research Collaboration
- 733 051 042/ZonMW Memorabel
- NIHR203312/National Institute for Health Research Cambridge Biomedical Research Centre
- MC_UU_00030/14/MRC_/Medical Research Council/United Kingdom
- MR/T033371/1/MRC_/Medical Research Council/United Kingdom
- Saul A. Silverman Family Foundation
- Canada International Scientific Exchange Program
- Morris Kerzner Memorial Fund
- 20/0448/Instituto de Salud Carlos III
- European Union
- University of Toronto Medical Science Open
- Joseph Bazylewicz Fellowships
- Swedish FTD Initiative Schörling Foundation
- Dnr 529-2014-7504/EU Joint Programme Neurodegenerative Disease Research Prefrontals Vetenskapsrådet
- EU Joint Programme Neurodegenerative Disease Research-GENFI-PROX
- 2019-0224/Vetenskapsrådet
- 2015-02926/Vetenskapsrådet
- 2018-02754/Vetenskapsrådet
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

