AutoDock-SS: AutoDock for Multiconformational Ligand-Based Virtual Screening
- PMID: 38624083
- PMCID: PMC11094722
- DOI: 10.1021/acs.jcim.4c00136
AutoDock-SS: AutoDock for Multiconformational Ligand-Based Virtual Screening
Abstract
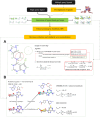
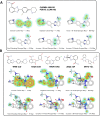
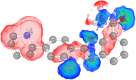
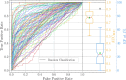
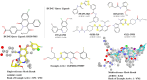
Ligand-based virtual screening (LBVS) can be pivotal for identifying potential drug leads, especially when the target protein's structure is unknown. However, current LBVS methods are limited in their ability to consider the ligand conformational flexibility. This study presents AutoDock-SS (Similarity Searching), which adapts protein-ligand docking for use in LBVS. AutoDock-SS integrates novel ligand-based grid maps and AutoDock-GPU into a novel three-dimensional LBVS workflow. Unlike other approaches based on pregenerated conformer libraries, AutoDock-SS's built-in conformational search optimizes conformations dynamically based on the reference ligand, thus providing a more accurate representation of relevant ligand conformations. AutoDock-SS supports two modes: single and multiple ligand queries, allowing for the seamless consideration of multiple reference ligands. When tested on the Directory of Useful Decoys─Enhanced (DUD-E) data set, AutoDock-SS surpassed alternative 3D LBVS methods, achieving a mean AUROC of 0.775 and an EF1% of 25.72 in single-reference mode. The multireference mode, evaluated on the augmented DUD-E+ data set, demonstrated superior accuracy with a mean AUROC of 0.843 and an EF1% of 34.59. This enhanced performance underscores AutoDock-SS's ability to treat compounds as conformationally flexible while considering the ligand's shape, pharmacophore, and electrostatic potential, expanding the potential of LBVS methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

