Peripheral HMGB1 is linked to O3 pathology of disease-associated astrocytes and amyloid
- PMID: 38624088
- PMCID: PMC11095433
- DOI: 10.1002/alz.13825
Peripheral HMGB1 is linked to O3 pathology of disease-associated astrocytes and amyloid
Abstract
Introduction: Ozone (O3) is an air pollutant associated with Alzheimer's disease (AD) risk. The lung-brain axis is implicated in O3-associated glial and amyloid pathobiology; however, the role of disease-associated astrocytes (DAAs) in this process remains unknown.
Methods: The O3-induced astrocyte phenotype was characterized in 5xFAD mice by spatial transcriptomics and proteomics. Hmgb1fl/fl LysM-Cre+ mice were used to assess the role of peripheral myeloid cell high mobility group box 1 (HMGB1).
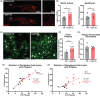
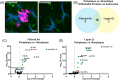
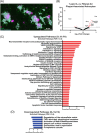
Results: O3 increased astrocyte and plaque numbers, impeded the astrocyte proteomic response to plaque deposition, augmented the DAA transcriptional fingerprint, increased astrocyte-microglia contact, and reduced bronchoalveolar lavage immune cell HMGB1 expression in 5xFAD mice. O3-exposed Hmgb1fl/fl LysM-Cre+ mice exhibited dysregulated DAA mRNA markers.
Discussion: Astrocytes and peripheral myeloid cells are critical lung-brain axis interactors. HMGB1 loss in peripheral myeloid cells regulates the O3-induced DAA phenotype. These findings demonstrate a mechanism and potential intervention target for air pollution-induced AD pathobiology.
Highlights: Astrocytes are part of the lung-brain axis, regulating how air pollution affects plaque pathology. Ozone (O3) astrocyte effects are associated with increased plaques and modified by plaque localization. O3 uniquely disrupts the astrocyte transcriptomic and proteomic disease-associated astrocyte (DAA) phenotype in plaque associated astrocytes (PAA). O3 changes the PAA cell contact with microglia and cell-cell communication gene expression. Peripheral myeloid cell high mobility group box 1 regulates O3-induced transcriptomic changes in the DAA phenotype.
Keywords: O3; amyloid plaques; disease‐associated astrocytes; lung–brain axis; peripheral HMGB1.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors have no competing interests to declare. Author disclosures are available in the supporting information.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

