Plasmodium yoelii surface-related antigen (PySRA) modulates the host pro-inflammatory responses via binding to CD68 on macrophage membrane
- PMID: 38624215
- PMCID: PMC11075460
- DOI: 10.1128/iai.00113-24
Plasmodium yoelii surface-related antigen (PySRA) modulates the host pro-inflammatory responses via binding to CD68 on macrophage membrane
Abstract
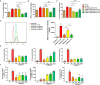
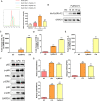
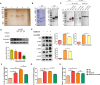
Malaria, one of the major infectious diseases in the world, is caused by the Plasmodium parasite. Plasmodium antigens could modulate the inflammatory response by binding to macrophage membrane receptors. As an export protein on the infected erythrocyte membrane, Plasmodium surface-related antigen (SRA) participates in the erythrocyte invasion and regulates the immune response of the host. This study found that the F2 segment of P. yoelii SRA activated downstream MAPK and NF-κB signaling pathways by binding to CD68 on the surface of the macrophage membrane and regulating the inflammatory response. The anti-PySRA-F2 antibody can protect mice against P. yoelii, and the pro-inflammatory responses such as IL-1β, TNF-α, and IL-6 after infection with P. yoelii are attenuated. These findings will be helpful for understanding the involvement of the pathogenic mechanism of malaria with the exported protein SRA.
Keywords: CD68; Plasmodium yoelii; inflammatory response; macrophage; surface-related antigen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Plasmodium yoelii Erythrocyte-Binding-like Protein Modulates Host Cell Membrane Structure, Immunity, and Disease Severity.mBio. 2020 Jan 7;11(1):e02995-19. doi: 10.1128/mBio.02995-19. mBio. 2020. PMID: 31911494 Free PMC article.
-
T-cell recognition of a cross-reactive antigen(s) in erythrocyte stages of Plasmodium falciparum and Plasmodium yoelii: inhibition of parasitemia by this antigen(s).Infect Immun. 1993 Nov;61(11):4863-9. doi: 10.1128/iai.61.11.4863-4869.1993. Infect Immun. 1993. PMID: 8104901 Free PMC article.
-
A malaria protein factor induces IL-4 production by dendritic cells via PI3K-Akt-NF-κB signaling independent of MyD88/TRIF and promotes Th2 response.J Biol Chem. 2018 Jul 6;293(27):10425-10434. doi: 10.1074/jbc.AC118.001720. Epub 2018 Apr 17. J Biol Chem. 2018. PMID: 29666186 Free PMC article.
-
The Plasmodium yoelii microgamete surface antigen (PyMiGS) induces anti-malarial transmission blocking immunity that reduces microgamete motility/release from activated male gametocytes.Vaccine. 2018 Nov 26;36(49):7463-7471. doi: 10.1016/j.vaccine.2018.10.067. Epub 2018 Oct 26. Vaccine. 2018. PMID: 30420038
-
Small variant surface antigens and Plasmodium evasion of immunity.Future Microbiol. 2010 Apr;5(4):663-82. doi: 10.2217/fmb.10.21. Future Microbiol. 2010. PMID: 20353305 Review.
References
-
- WHO . 2022. World malaria report (2022). World Health Organization, Geneva.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

