Application of quinoline derivatives in third-generation photovoltaics
- PMID: 38624760
- PMCID: PMC8267773
- DOI: 10.1007/s10854-021-06225-6
Application of quinoline derivatives in third-generation photovoltaics
Abstract
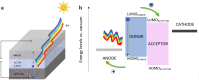
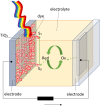
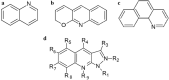
Among many chemical compounds synthesized for third-generation photovoltaic applications, quinoline derivatives have recently gained popularity. This work reviews the latest developments in the quinoline derivatives (metal complexes) for applications in the photovoltaic cells. Their properties for photovoltaic applications are detailed: absorption spectra, energy levels, and other achievements presented by the authors. We have also outlined various methods for testing the compounds for application. Finally, we present the implementation of quinoline derivatives in photovoltaic cells. Their architecture and design are described, and also, the performance for polymer solar cells and dye-synthesized solar cells was highlighted. We have described their performance and characteristics. We have also pointed out other, non-photovoltaic applications for quinoline derivatives. It has been demonstrated and described that quinoline derivatives are good materials for the emission layer of organic light-emitting diodes (OLEDs) and are also used in transistors. The compounds are also being considered as materials for biomedical applications.
© The Author(s) 2021.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures

Similar articles
-
Polymer Materials for Optoelectronics and Energy Applications.Materials (Basel). 2024 Jul 26;17(15):3698. doi: 10.3390/ma17153698. Materials (Basel). 2024. PMID: 39124361 Free PMC article. Review.
-
Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review.Polymers (Basel). 2020 Nov 9;12(11):2627. doi: 10.3390/polym12112627. Polymers (Basel). 2020. PMID: 33182241 Free PMC article. Review.
-
Novel Na(+) doped Alq3 hybrid materials for organic light-emitting diode (OLED) devices and flat panel displays.Luminescence. 2015 May;30(3):251-6. doi: 10.1002/bio.2721. Epub 2014 Jul 18. Luminescence. 2015. PMID: 25045087
-
New 3pi-2spiro ladder-type phenylene materials: synthesis, physicochemical properties and applications in OLEDs.Chemistry. 2008;14(36):11328-42. doi: 10.1002/chem.200801428. Chemistry. 2008. PMID: 19009573
-
Highly Efficient 7,7-Dimethyl-9-(10-phenylanthracen-9-yl)-7H-Benzo[6,7]Indeno[1,2-f]Quinoline Derivatives for Blue Fluorescent Organic Light-Emitting Diodes.J Nanosci Nanotechnol. 2021 Aug 1;21(8):4341-4346. doi: 10.1166/jnn.2021.19400. J Nanosci Nanotechnol. 2021. PMID: 33714325
Cited by
-
Copper-promoted oxidative mono- and di-bromination of 8-aminoquinoline amides with HBr and DMSO.RSC Adv. 2025 Mar 21;15(11):8750-8756. doi: 10.1039/d5ra00492f. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40124911 Free PMC article.
-
Microwave-assisted protocol towards synthesis of heterocyclic molecules: a comparative analysis with conventional synthetic methodologies (years 2019-2023): a review.Mol Divers. 2025 Jun;29(3):2717-2763. doi: 10.1007/s11030-024-10981-y. Epub 2024 Sep 20. Mol Divers. 2025. PMID: 39302538 Review.
-
Synthesis of novel sulphonamide derivatives from tunable quinolines with computational studies.Sci Rep. 2025 Mar 31;15(1):10972. doi: 10.1038/s41598-025-94817-1. Sci Rep. 2025. PMID: 40164813 Free PMC article.
-
Advances in Mechanochemical Methods for One-Pot Multistep Organic Synthesis.Chemistry. 2025 Jun 17;31(34):e202500798. doi: 10.1002/chem.202500798. Epub 2025 May 24. Chemistry. 2025. PMID: 40327599 Free PMC article. Review.
-
Through-Space Conjugated Electron Transport Materials for Improving Efficiency and Lifetime of Organic Light-Emitting Diodes.Adv Sci (Weinh). 2022 May;9(15):e2200374. doi: 10.1002/advs.202200374. Epub 2022 Mar 24. Adv Sci (Weinh). 2022. PMID: 35322599 Free PMC article.
References
-
- B. Pandey, M. Gautam, M. Agrawal, Greenhouse Gas Emissions from Coal Mining Activities and Their Possible Mitigation Strategies (Elsevier Inc., Amsterdam, 2017).
-
- L. Haelg, Energy Res. Soc. Sci. 69, 101636 (2020)
-
- J.Z. Thellufsen, H. Lund, S. Nielse, P. Sorknæs, S.R. Djørup, K. Sperling, P.A. Østergaard, D.W. Drysdale, M. Chang, in Proceedings of the 14th Conference on Sustainable Development of Energy, Water and Environment Systems, vol. 129 (2019)
-
- G. Gozgor, M.K. Mahalik, E. Demir, H. Padhan, Energy Policy 139, 111365 (2020)
Publication types
LinkOut - more resources
Full Text Sources
