Antibacterial agents used in COVID-19: A systematic review and meta-analysis
- PMID: 38624829
- PMCID: PMC8181540
- DOI: 10.1007/s42398-021-00194-6
Antibacterial agents used in COVID-19: A systematic review and meta-analysis
Abstract
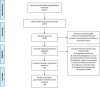
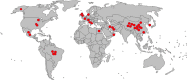
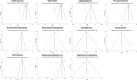
There have been speculations regarding rise in antimicrobial resistance (AMR) globally owing to indiscriminate antibiotic usage during the COVID-19 pandemic. To curb the menace through decisive policies, it is essential to assess the antibiotics, particularly the antibacterial agents. This systematic review and meta-analysis were performed to assess antibiotic use in COVID-19 patients. A thorough systematic search was undertaken in databases like PubMed, Cochrane library, Google Scholar, World Health Organization (WHO) database and clinicaltrials.gov by two independent reviewers for articles in English published from January 1, 2019 to October 31, 2020. Studies were included if they assessed confirmed COVID-19 cases and mentioned the use of antibiotics. The primary outcome was the proportion of COVID-19 patients subjected to specific antibacterial agents. An attempt to stratify the data based on study settings and disease severity was also performed. Of the total 6012 studies screened, 40 were eligible for qualitative review and 19 for meta-analysis. Specific antibacterial agents were mentioned in 23 studies (57.5%). In the random effect meta-analysis, pooled prevalence of azithromycin use was 24.5% (95% CI 22.9-26.2%) followed by cephalosporins as 26.6% (95% CI 24.9-28.4). None of the studies clearly specified indications for antibiotic use. Ten studies (25%) mentioned empirical use of antibiotics. Bacterial co-infections/secondary infections were documented in four studies with mean prevalence of infection of 1.9% (95% CI 1.2-2.8%). There is lack of data on use of specific antibacterial agents, indications for their use based on severity of infections and microbiological evidence of bacterial co-infections.
Keywords: Antibiotics; Antimicrobial resistance; Azithromycin; Bacterial co-infections; COVID-19; Cephalosporins; Empirical.
© Society for Environmental Sustainability 2021.
Conflict of interest statement
Conflict of interestAll authors declare that they have no conflict of interest.
Figures


References
-
- An MH, Kim MS, Kim BO, Kang SH, Kimn WJ, Park SK, Park HW, Yang W et al (2020) Treatment response to hydroxychloroquine and antibiotics for mild to moderate COVID-19: a retrospective cohort study from South Korea. medRxiv. 10.1101/2020.07.04.20146548
-
- Banerjee T, Anupurba S, Singh DK (2013) Poor compliance with the antibiotic policy in the intensive care unit (ICU) of a tertiary care hospital in India. J Infect Dev Ctries 15:7 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
