Cadherin-17 as a target for the immunoPET of adenocarcinoma
- PMID: 38625402
- PMCID: PMC11223962
- DOI: 10.1007/s00259-024-06709-7
Cadherin-17 as a target for the immunoPET of adenocarcinoma
Abstract
Purpose: Cadherin-17 (CDH17) is a calcium-dependent cell adhesion protein that is overexpressed in several adenocarcinomas, including gastric, colorectal, and pancreatic adenocarcinoma. High levels of CDH17 have been linked to metastatic disease and poor prognoses in patients with these malignancies, fueling interest in the protein as a target for diagnostics and therapeutics. Herein, we report the synthesis, in vitro validation, and in vivo evaluation of a CDH17-targeted 89Zr-labeled immunoPET probe.
Methods: The CDH17-targeting mAb D2101 was modified with an isothiocyanate-bearing derivative of desferrioxamine (DFO) to produce a chelator-bearing immunoconjugate - DFO-D2101 - and flow cytometry and surface plasmon resonance (SPR) were used to interrogate its antigen-binding properties. The immunoconjugate was then radiolabeled with zirconium-89 (t1/2 ~ 3.3 days), and the serum stability and immunoreactive fraction of [89Zr]Zr-DFO-D2101 were determined. Finally, [89Zr]Zr-DFO-D2101's performance was evaluated in a trio of murine models of pancreatic ductal adenocarcinoma (PDAC): subcutaneous, orthotopic, and patient-derived xenografts (PDX). PET images were acquired over the course of 5 days, and terminal biodistribution data were collected after the final imaging time point.
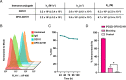
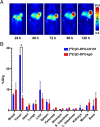
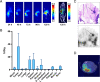
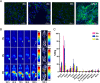
Results: DFO-D2101 was produced with a degree of labeling of ~ 1.1 DFO/mAb. Flow cytometry with CDH17-expressing AsPC-1 cells demonstrated that the immunoconjugate binds to its target in a manner similar to its parent mAb, while SPR with recombinant CDH17 revealed that D2101 and DFO-D2101 exhibit nearly identical KD values: 8.2 × 10-9 and 6.7 × 10-9 M, respectively. [89Zr]Zr-DFO-D2101 was produced with a specific activity of 185 MBq/mg (5.0 mCi/mg), remained >80% stable in human serum over the course of 5 days, and boasted an immunoreactive fraction of >0.85. In all three murine models of PDAC, the radioimmunoconjugate yielded high contrast images, with high activity concentrations in tumor tissue and low uptake in non-target organs. Tumoral activity concentrations reached as high as >60 %ID/g in two of the cohorts bearing PDXs.
Conclusion: Taken together, these data underscore that [89Zr]Zr-DFO-D2101 is a highly promising probe for the non-invasive visualization of CDH17 expression in PDAC. We contend that this radioimmunoconjugate could have a significant impact on the clinical management of patients with both PDAC and gastrointestinal adenocarcinoma, most likely as a theranostic imaging tool in support of CDH17-targeted therapies.
Keywords: Adenocarcinoma; Cadherin-17; Pancreatic ductal adenocarcinoma; Positron emission tomography; Radioimmunoconjugate; Zirconium-89.
© 2024. The Author(s).
Conflict of interest statement
TK is a founder and representative director of PhotoQ3, Inc. The other authors have no relevant financial or non-financial interests to disclose.
Figures
References
MeSH terms
Substances
Grants and funding
- R01CA240963/Foundation for the National Institutes of Health
- R01 CA240963/CA/NCI NIH HHS/United States
- R00ES034053/Foundation for the National Institutes of Health
- 1R01CA244327/Foundation for the National Institutes of Health
- 1R01AI175417/Foundation for the National Institutes of Health
- P30 CA008748/CA/NCI NIH HHS/United States
- R00CA226363/Foundation for the National Institutes of Health
- R01 CA244327/CA/NCI NIH HHS/United States
- R01 CA281801/CA/NCI NIH HHS/United States
- R01 CA204167/CA/NCI NIH HHS/United States
- R01 AI175417/AI/NIAID NIH HHS/United States
- 1R01CA281801/Foundation for the National Institutes of Health
- F31CA275334/Foundation for the National Institutes of Health
LinkOut - more resources
Full Text Sources

