Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency
- PMID: 38625743
- PMCID: PMC11141923
- DOI: 10.1172/jci.insight.168594
Altered lipid homeostasis is associated with cerebellar neurodegeneration in SNX14 deficiency
Abstract
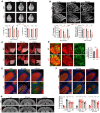
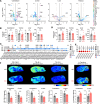
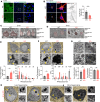
Dysregulated lipid homeostasis is emerging as a potential cause of neurodegenerative disorders. However, evidence of errors in lipid homeostasis as a pathogenic mechanism of neurodegeneration remains limited. Here, we show that cerebellar neurodegeneration caused by Sorting Nexin 14 (SNX14) deficiency is associated with lipid homeostasis defects. Recent studies indicate that SNX14 is an interorganelle lipid transfer protein that regulates lipid transport, lipid droplet (LD) biogenesis, and fatty acid desaturation, suggesting that human SNX14 deficiency belongs to an expanding class of cerebellar neurodegenerative disorders caused by altered cellular lipid homeostasis. To test this hypothesis, we generated a mouse model that recapitulates human SNX14 deficiency at a genetic and phenotypic level. We demonstrate that cerebellar Purkinje cells (PCs) are selectively vulnerable to SNX14 deficiency while forebrain regions preserve their neuronal content. Ultrastructure and lipidomic studies reveal widespread lipid storage and metabolism defects in SNX14-deficient mice. However, predegenerating SNX14-deficient cerebella show a unique accumulation of acylcarnitines and depletion of triglycerides. Furthermore, defects in LD content and telolysosome enlargement in predegenerating PCs suggest lipotoxicity as a pathogenic mechanism of SNX14 deficiency. Our work shows a selective cerebellar vulnerability to altered lipid homeostasis and provides a mouse model for future therapeutic studies.
Keywords: Lysosomes; Monogenic diseases; Neurodegeneration; Neuroscience.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

