SIRT3 regulates cardiolipin biosynthesis in pressure overload-induced cardiac remodeling by PPARγ-mediated mechanism
- PMID: 38625851
- PMCID: PMC11020683
- DOI: 10.1371/journal.pone.0301990
SIRT3 regulates cardiolipin biosynthesis in pressure overload-induced cardiac remodeling by PPARγ-mediated mechanism
Abstract
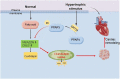
Cardiac remodeling is the primary pathological feature of chronic heart failure (HF). Exploring the characteristics of cardiac remodeling in the very early stages of HF and identifying targets for intervention are essential for discovering novel mechanisms and therapeutic strategies. Silent mating type information regulation 2 homolog 3 (SIRT3), as a major mitochondrial nicotinamide adenine dinucleotide (NAD)-dependent deacetylase, is required for mitochondrial metabolism. However, whether SIRT3 plays a role in cardiac remodeling by regulating the biosynthesis of mitochondrial cardiolipin (CL) is unknown. In this study, we induced pressure overload in wild-type (WT) and SIRT3 knockout (SIRT3-/-) mice via transverse aortic constriction (TAC). Compared with WT mouse hearts, the hearts of SIRT3-/- mice exhibited more-pronounced cardiac remodeling and fibrosis, greater reactive oxygen species (ROS) production, decreased mitochondrial-membrane potential (ΔΨm), and abnormal mitochondrial morphology after TAC. Furthermore, SIRT3 deletion aggravated TAC-induced decrease in total CL content, which might be associated with the downregulation of the CL synthesis related enzymes cardiolipin synthase 1 (CRLS1) and phospholipid-lysophospholipid transacylase (TAFAZZIN). In our in vitro experiments, SIRT3 overexpression prevented angiotensin II (AngII)- induced aberrant mitochondrial function, CL biosynthesis disorder, and peroxisome proliferator-activated receptor gamma (PPARγ) downregulation in cardiomyocytes; meanwhile, SIRT3 knockdown exacerbated these effects. Moreover, the addition of GW9662, a PPARγ antagonist, partially counteracted the beneficial effects of SIRT3 overexpression. In conclusion, SIRT3 regulated PPARγ-mediated CL biosynthesis, maintained the structure and function of mitochondria, and thereby protected the myocardium against cardiac remodeling.
Copyright: © 2024 Liu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Sirtuin 3 Deficiency Accelerates Hypertensive Cardiac Remodeling by Impairing Angiogenesis.J Am Heart Assoc. 2017 Aug 19;6(8):e006114. doi: 10.1161/JAHA.117.006114. J Am Heart Assoc. 2017. PMID: 28862956 Free PMC article.
-
Sirtuin-3 (SIRT3) Protein Attenuates Doxorubicin-induced Oxidative Stress and Improves Mitochondrial Respiration in H9c2 Cardiomyocytes.J Biol Chem. 2015 Apr 24;290(17):10981-93. doi: 10.1074/jbc.M114.607960. Epub 2015 Mar 10. J Biol Chem. 2015. PMID: 25759382 Free PMC article.
-
NMNAT3 is involved in the protective effect of SIRT3 in Ang II-induced cardiac hypertrophy.Exp Cell Res. 2016 Oct 1;347(2):261-73. doi: 10.1016/j.yexcr.2016.07.006. Epub 2016 Jul 14. Exp Cell Res. 2016. PMID: 27423420
-
Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy.Biochim Biophys Acta Mol Basis Dis. 2017 Aug;1863(8):1973-1983. doi: 10.1016/j.bbadis.2016.10.021. Epub 2016 Oct 26. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27794418 Review.
-
Cardiolipin remodeling and the function of tafazzin.Biochim Biophys Acta. 2013 Mar;1831(3):582-8. doi: 10.1016/j.bbalip.2012.11.007. Epub 2012 Nov 28. Biochim Biophys Acta. 2013. PMID: 23200781 Review.
References
-
- Bedi KC, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, et al.. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133(8):706–16. doi: 10.1161/CIRCULATIONAHA.115.017545 . - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

