Integrated mutational landscape analysis of poorly differentiated high-grade neuroendocrine carcinoma of the uterine cervix
- PMID: 38625939
- PMCID: PMC11046577
- DOI: 10.1073/pnas.2321898121
Integrated mutational landscape analysis of poorly differentiated high-grade neuroendocrine carcinoma of the uterine cervix
Abstract
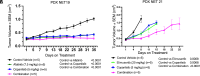
High-grade neuroendocrine cervical cancers (NETc) are exceedingly rare, highly aggressive tumors. We analyzed 64 NETc tumor samples by whole-exome sequencing (WES). Human papillomavirus DNA was detected in 65.6% (42/64) of the tumors. Recurrent mutations were identified in PIK3CA, KMT2D/MLL2, K-RAS, ARID1A, NOTCH2, and RPL10. The top mutated genes included RB1, ARID1A, PTEN, KMT2D/MLL2, and WDFY3, a gene not yet implicated in NETc. Somatic CNV analysis identified two copy number gains (3q27.1 and 19q13.12) and five copy number losses (1p36.21/5q31.3/6p22.2/9q21.11/11p15.5). Also, gene fusions affecting the ACLY-CRHR1 and PVT1-MYC genes were identified in one of the eight samples subjected to RNA sequencing. To resolve evolutionary history, multiregion WES in NETc admixed with adenocarcinoma cells was performed (i.e., mixed-NETc). Phylogenetic analysis of mixed-NETc demonstrated that adenocarcinoma and neuroendocrine elements derive from a common precursor with mutations typical of adenocarcinomas. Over one-third (22/64) of NETc demonstrated a mutator phenotype of C > T at CpG consistent with deficiencies in MBD4, a member of the base excision repair (BER) pathway. Mutations in the PI3K/AMPK pathways were identified in 49/64 samples. We used two patient-derived-xenografts (PDX) (i.e., NET19 and NET21) to evaluate the activity of pan-HER (afatinib), PIK3CA (copanlisib), and ATR (elimusertib) inhibitors, alone and in combination. PDXs harboring alterations in the ERBB2/PI3K/AKT/mTOR/ATR pathway were sensitive to afatinib, copanlisib, and elimusertib (P < 0.001 vs. controls). However, combinations of copanlisib/afatinib and copanlisib/elimusertib were significantly more effective in controlling NETc tumor growth. These findings define the genetic landscape of NETc and suggest that a large subset of these highly lethal malignancies might benefit from existing targeted therapies.
Keywords: cancer; genetic analysis; mutations; neuroendocrine; oncogene.
Conflict of interest statement
Competing interests statement:A.D.S. is listed as author in a paper (i.e.,
Figures




References
-
- Gadducci A., Carinelli S., Aletti G., Neuroendrocrine tumors of the uterine cervix: A therapeutic challenge for gynecologic oncologists. Gynecol. Oncol. 144, 637–646 (2017). - PubMed
-
- McCusker M. E., Coté T. R., Clegg L. X., Tavassoli F. J., Endocrine tumors of the uterine cervix: Incidence, demographics, and survival with comparison to squamous cell carcinoma. Gynecol. Oncol. 88, 333–339 (2003). - PubMed
-
- Pei X., et al. , The next generation sequencing of cancer-related genes in small cell neuroendocrine carcinoma of the cervix. Gynecol. Oncol. 161, 779–786 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

