Evidence for vagal sensory neural involvement in influenza pathogenesis and disease
- PMID: 38626267
- PMCID: PMC11051609
- DOI: 10.1371/journal.ppat.1011635
Evidence for vagal sensory neural involvement in influenza pathogenesis and disease
Abstract
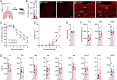
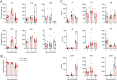
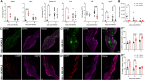
Influenza A virus (IAV) is a common respiratory pathogen and a global cause of significant and often severe morbidity. Although inflammatory immune responses to IAV infections are well described, little is known about how neuroimmune processes contribute to IAV pathogenesis. In the present study, we employed surgical, genetic, and pharmacological approaches to manipulate pulmonary vagal sensory neuron innervation and activity in the lungs to explore potential crosstalk between pulmonary sensory neurons and immune processes. Intranasal inoculation of mice with H1N1 strains of IAV resulted in stereotypical antiviral lung inflammation and tissue pathology, changes in breathing, loss of body weight and other clinical signs of severe IAV disease. Unilateral cervical vagotomy and genetic ablation of pulmonary vagal sensory neurons had a moderate effect on the pulmonary inflammation induced by IAV infection, but significantly worsened clinical disease presentation. Inhibition of pulmonary vagal sensory neuron activity via inhalation of the charged sodium channel blocker, QX-314, resulted in a moderate decrease in lung pathology, but again this was accompanied by a paradoxical worsening of clinical signs. Notably, vagal sensory ganglia neuroinflammation was induced by IAV infection and this was significantly potentiated by QX-314 administration. This vagal ganglia hyperinflammation was characterized by alterations in IAV-induced host defense gene expression, increased neuropeptide gene and protein expression, and an increase in the number of inflammatory cells present within the ganglia. These data suggest that pulmonary vagal sensory neurons play a role in the regulation of the inflammatory process during IAV infection and suggest that vagal neuroinflammation may be an important contributor to IAV pathogenesis and clinical presentation. Targeting these pathways could offer therapeutic opportunities to treat IAV-induced morbidity and mortality.
Copyright: © 2024 Verzele et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

