Recent advances in immunotherapies against infectious diseases
- PMID: 38626281
- PMCID: PMC7717302
- DOI: 10.1093/immadv/ltaa007
Recent advances in immunotherapies against infectious diseases
Abstract
Immunotherapies are disease management strategies that target or manipulate components of the immune system. Infectious diseases pose a significant threat to human health as evidenced by countries continuing to grapple with several emerging and re-emerging diseases, the most recent global health threat being the SARS-CoV2 pandemic. As such, various immunotherapeutic approaches are increasingly being investigated as alternative therapies for infectious diseases, resulting in significant advances towards the uncovering of pathogen-host immunity interactions. Novel and innovative therapeutic strategies are necessary to overcome the challenges typically faced by existing infectious disease prevention and control methods such as lack of adequate efficacy, drug toxicity, and the emergence of drug resistance. As evidenced by recent developments and success of pharmaceuticals such as monoclonal antibodies (mAbs), immunotherapies already show abundant promise to overcome such limitations while also advancing the frontiers of medicine. In this review, we summarize some of the most notable inroads made to combat infectious disease, over mainly the last 5 years, through the use of immunotherapies such as vaccines, mAb-based therapies, T-cell-based therapies, manipulation of cytokine levels, and checkpoint inhibition. While its most general applications are founded in cancer treatment, advances made towards the curative treatment of human immunodeficiency virus, tuberculosis, malaria, zika virus and, most recently COVID-19, reinforce the role of immunotherapeutic strategies in the broader field of disease control. Ultimately, the comprehensive specificity, safety, and cost of immunotherapeutics will impact its widespread implementation.
Keywords: T-cells; checkpoint inhibition; immunotherapy; infectious disease; vaccine.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures
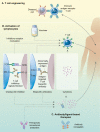
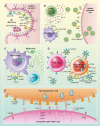
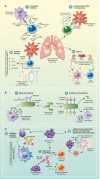
References
-
- Janeway CA Jr, Travers P, Walport M et al. Immunobiology: The Immune System in Health and Disease. Immunological Memory. 5th edn. New York: Garland Science; 2001.
-
- Hooks MA, Wade CS, Millikan WJ Jr. Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy 1991;11:26–37. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
