Recessively Inherited Deficiency of Secreted WFDC2 (HE4) Causes Nasal Polyposis and Bronchiectasis
- PMID: 38626355
- PMCID: PMC11197063
- DOI: 10.1164/rccm.202308-1370OC
Recessively Inherited Deficiency of Secreted WFDC2 (HE4) Causes Nasal Polyposis and Bronchiectasis
Abstract
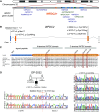
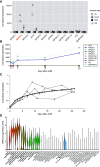
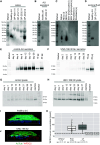
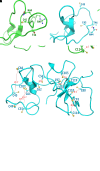
Rationale: Bronchiectasis is a pathological dilatation of the bronchi in the respiratory airways associated with environmental or genetic causes (e.g., cystic fibrosis, primary ciliary dyskinesia, and primary immunodeficiency disorders), but most cases remain idiopathic. Objectives: To identify novel genetic defects in unsolved cases of bronchiectasis presenting with severe rhinosinusitis, nasal polyposis, and pulmonary Pseudomonas aeruginosa infection. Methods: DNA was analyzed by next-generation or targeted Sanger sequencing. RNA was analyzed by quantitative PCR and single-cell RNA sequencing. Patient-derived cells, cell cultures, and secretions (mucus, saliva, seminal fluid) were analyzed by Western blotting and immunofluorescence microscopy, and mucociliary activity was measured. Blood serum was analyzed by electrochemiluminescence immunoassay. Protein structure and proteomic analyses were used to assess the impact of a disease-causing founder variant. Measurements and Main Results: We identified biallelic pathogenic variants in WAP four-disulfide core domain 2 (WFDC2) in 11 individuals from 10 unrelated families originating from the United States, Europe, Asia, and Africa. Expression of WFDC2 was detected predominantly in secretory cells of control airway epithelium and also in submucosal glands. We demonstrate that WFDC2 is below the limit of detection in blood serum and hardly detectable in samples of saliva, seminal fluid, and airway surface liquid from WFDC2-deficient individuals. Computer simulations and deglycosylation assays indicate that the disease-causing founder variant p.Cys49Arg structurally hampers glycosylation and, thus, secretion of mature WFDC2. Conclusions: WFDC2 dysfunction defines a novel molecular etiology of bronchiectasis characterized by the deficiency of a secreted component of the airways. A commercially available blood test combined with genetic testing allows its diagnosis.
Keywords: Pseudomonas; chronic airway disease; infertility.
Figures

Comment in
-
Deciphering Idiopathic Bronchiectasis One Gene at a Time.Am J Respir Crit Care Med. 2024 Jul 1;210(1):12-13. doi: 10.1164/rccm.202403-0611ED. Am J Respir Crit Care Med. 2024. PMID: 38717359 Free PMC article. No abstract available.
References
-
- Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J . 2017;50:1700629. - PubMed
-
- Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respir Med . 2007;101:1163–1170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 HG006504/HG/NHGRI NIH HHS/United States
- U2C TR002818/TR/NCATS NIH HHS/United States
- U54 HL096458/HL/NHLBI NIH HHS/United States
- R01 HL117836/HL/NHLBI NIH HHS/United States
- R01 HL071798/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P30 DK065988/DK/NIDDK NIH HHS/United States
- OM6/7-2/Deutsche Forschungsgemeinschaft
- OM6/8-2/Deutsche Forschungsgemeinschaft
- OM6/10-1/Deutsche Forschungsgemeinschaft
- CRU 326/Deutsche Forschungsgemeinschaft
- OL450/3-1/Deutsche Forschungsgemeinschaft
- RA3522/1-1/Deutsche Forschungsgemeinschaft
- HJ 7/1-1/Deutsche Forschungsgemeinschaft
- HJ 7/1-3/Deutsche Forschungsgemeinschaft
- Om2/009/12/Interdisziplinäres Zentrum für klinische Forschung Münster (IZKF)
- Om2/015/16/Interdisziplinäres Zentrum für klinische Forschung Münster (IZKF)
- Om2/010/20/Interdisziplinäres Zentrum für klinische Forschung Münster (IZKF)
- Horizon2020 GA 777295/European Commission Registry Warehouse
- EU FP7 GA 305404/BESTCILIA
- Tistou and Charlotte Kerstan Stiftung
- 210585/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- U54HL096458/US NIH/ Office of Rare Diseases Research/National Center for Advancing Translational Sciences (NCATS)/National Heart, Lung, and Blood Institute
- R01HL071798/US NIH
- R01HL117836/US NIH
- X01HL115246-01/US NIH
- 82070003/National Natural Science Foundation of China
- 82270048/National Natural Science Foundation of China
- 91967/Mayo Foundation for Medical Education and Research
- 21EQUI09Z6RCHX/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- ANR-19-P3IA-0002/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- ANR-19-CE14-0027/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- ANR-19-P3IA-0002-3IA/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- ANR-21-ESRE-0052/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- ANR-10-INBS-09-03/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- ANR-10-INBS-09-02/CNRS, Inserm, the infrastructure France Génomique and the French Government (Agence Nationale de Recherche, ANR)
- 2017-175159-5022/Canceropôle PACA, the H2020 Health (Discovair) and the Chan Zuckerberg Initiative
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

