Obstetric Complications and Birth Outcomes After Antenatal Coronavirus Disease 2019 (COVID-19) Vaccination
- PMID: 38626447
- PMCID: PMC11090513
- DOI: 10.1097/AOG.0000000000005583
Obstetric Complications and Birth Outcomes After Antenatal Coronavirus Disease 2019 (COVID-19) Vaccination
Abstract
Objective: To evaluate the association between antenatal messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccination and risk of adverse pregnancy outcomes.
Methods: This was a retrospective cohort study of individuals with singleton pregnancies with live deliveries between June 1, 2021, and January 31, 2022, with data available from eight integrated health care systems in the Vaccine Safety Datalink. Vaccine exposure was defined as receipt of one or two mRNA COVID-19 vaccine doses (primary series) during pregnancy. Outcomes were preterm birth (PTB) before 37 weeks of gestation, small-for-gestational age (SGA) neonates, gestational diabetes mellitus (GDM), gestational hypertension, and preeclampsia-eclampsia-HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Outcomes in individuals vaccinated were compared with those in propensity-matched individuals with unexposed pregnancies. Adjusted hazard ratios (aHRs) and 95% CIs were estimated for PTB and SGA using a time-dependent covariate Cox model, and adjusted relative risks (aRRs) were estimated for GDM, gestational hypertension, and preeclampsia-eclampsia-HELLP syndrome using Poisson regression with robust variance.
Results: Among 55,591 individuals eligible for inclusion, 23,517 (42.3%) received one or two mRNA COVID-19 vaccine doses during pregnancy. Receipt of mRNA COVID-19 vaccination varied by maternal age, race, Hispanic ethnicity, and history of COVID-19. Compared with no vaccination, mRNA COVID-19 vaccination was associated with a decreased risk of PTB (rate: 6.4 [vaccinated] vs 7.7 [unvaccinated] per 100, aHR 0.89; 95% CI, 0.83-0.94). Messenger RNA COVID-19 vaccination was not associated with SGA (8.3 vs 7.4 per 100; aHR 1.06, 95% CI, 0.99-1.13), GDM (11.9 vs 10.6 per 100; aRR 1.00, 95% CI, 0.90-1.10), gestational hypertension (10.8 vs 9.9 per 100; aRR 1.08, 95% CI, 0.96-1.22), or preeclampsia-eclampsia-HELLP syndrome (8.9 vs 8.4 per 100; aRR 1.10, 95% CI, 0.97-1.24).
Conclusion: Receipt of an mRNA COVID-19 vaccine during pregnancy was not associated with an increased risk of adverse pregnancy outcomes; this information will be helpful for patients and clinicians when considering COVID-19 vaccination in pregnancy.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Financial Disclosure Kimberly K. Vesco's travel to the American Diabetes Association Meeting to present the research was covered by grant funds from the CDC. She received an Independent Grant for Learning and Change (Grant # 42360015), funded by Pfizer, Inc., and paid to her institution, to develop a novel menopause curriculum for medical residents. Darios Getahun's institution received funding from Hologic, Inc. and Johnson & Johnson. Candace C. Fuller is employed at Harvard Pilgrim Health Care institute, a non-profit organization that conducts work for government and private organizations, including pharmaceutical companies. Gabriela Vazquez-Benitez has received research funding from Sanofi Pasteur and AbbVie for unrelated work. Thomas Boyce has received grants from Pfizer, Moderna, and GlaxoSmithKline for unrelated research. The other authors did not report any potential conflicts of interest.
Figures
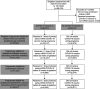
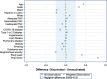
References
-
- Gurol-Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol 2021;225:522 e1–11. doi: 10.1016/j.ajog.2021.05.016 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding. Accessed February 15, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregn...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

