Hepatic steatosis modeling and MRI signal simulations for comparison of single- and dual-R2* models and estimation of fat fraction at 1.5T and 3T
- PMID: 38626508
- PMCID: PMC12149756
- DOI: 10.1016/j.compbiomed.2024.108448
Hepatic steatosis modeling and MRI signal simulations for comparison of single- and dual-R2* models and estimation of fat fraction at 1.5T and 3T
Abstract
Background and objective: Magnetic resonance imaging (MRI) has emerged as a noninvasive clinical tool for assessment of hepatic steatosis. Multi-spectral fat-water MRI models, incorporating single or dual transverse relaxation decay rate(s) (R2*) have been proposed for accurate fat fraction (FF) estimation. However, it is still unclear whether single- or dual-R2* model accurately mimics in vivo signal decay for precise FF estimation and the impact of signal-to-noise ratio (SNR) on each model performance. Hence, this study aims to construct virtual steatosis models and synthesize MRI signals with different SNRs to systematically evaluate the accuracy of single- and dual-R2* models for FF and R2* estimations at 1.5T and 3.0T.
Methods: Realistic hepatic steatosis models encompassing clinical FF range (0-60 %) were created using morphological features of fat droplets (FDs) extracted from human liver biopsy samples. MRI signals were synthesized using Monte Carlo simulations for noise-free (SNRideal) and varying SNR conditions (5-100). Fat-water phantoms were scanned with different SNRs to validate simulation results. Fat water toolbox was used to calculate R2* and FF for both single- and dual-R2* models. The model accuracies in R2* and FF estimates were analyzed using linear regression, bias plot and heatmap analysis.
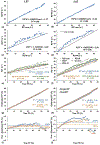
Results: The virtual steatosis model closely mimicked in vivo fat morphology and Monte Carlo simulation produced realistic MRI signals. For SNRideal and moderate-high SNRs, water R2* (R2*W) by dual-R2* and common R2* (R2*com) by single-R2* model showed an excellent agreement with slope close to unity (0.95-1.01) and R2 > 0.98 at both 1.5T and 3.0T. In simulations, the R2*com-FF and R2*W-FF relationships exhibited slopes similar to in vivo calibrations, confirming the accuracy of our virtual models. For SNRideal, fat R2* (R2*F) was similar to R2*W and dual-R2* model showed slightly higher accuracy in FF estimation. However, in the presence of noise, dual-R2* produced higher FF bias with decreasing SNR, while leading to only marginal improvement for high SNRs and in regions dominated by fat and water. In contrast, single-R2* model was robust and produced accurate FF estimations in simulations and phantom scans with clinical SNRs.
Conclusion: Our study demonstrates the feasibility of creating virtual steatosis models and generating MRI signals that mimic in vivo morphology and signal behavior. The single-R2* model consistently produced lower FF bias for clinical SNRs across entire FF range compared to dual-R2* model, hence signifying that single-R2* model is optimal for assessing hepatic steatosis.
Keywords: Fat fraction; Hepatic steatosis; Liver; Monte Carlo; R2*; Relaxometry; Simulation.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ, The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases, Hepatology, 67 (2018) 328–357. - PubMed
-
- Chen Y-Y, Yeh MM, Non-alcoholic fatty liver disease: A review with clinical and pathological correlation, Journal of the Formosan Medical Association, 120 (2021) 68–77. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M, Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes, Hepatology, 64 (2016) 73–84. - PubMed
-
- Vernon G, Baranova A, Younossi ZM, Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults, Alimentary Pharmacology & Therapeutics, 34 (2011) 274–285. - PubMed
-
- Angulo P, Nonalcoholic Fatty Liver Disease, New England Journal of Medicine, 346 (2002) 1221–1231. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

