Effect of Non-Rotavirus Enteric Infections on Vaccine Efficacy in a ROTASIIL Clinical Trial
- PMID: 38626750
- PMCID: PMC11154053
- DOI: 10.4269/ajtmh.23-0348
Effect of Non-Rotavirus Enteric Infections on Vaccine Efficacy in a ROTASIIL Clinical Trial
Abstract
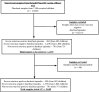
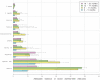
This study examined the relative proportion of enteric pathogens associated with severe gastroenteritis (GE) among children younger than 2 years in a phase III efficacy trial of the ROTASIIL® vaccine in India, evaluated the impact of co-infections on vaccine efficacy (VE), and characterized the association between specific pathogens and the clinical profile of severe GE. Stored stool samples collected from cases of severe GE in the phase III trial were tested by quantitative polymerase chain reaction using TaqMan™ Array Cards. Etiology was attributed by calculating the adjusted attributable fraction (AF) for each pathogen. A test-negative design was used to estimate VE. The pathogens with the highest AFs for severe diarrhea were rotavirus (23.5%), adenovirus 40/41 (17.0%), Shigella spp./enteroinvasive Escherichia coli, norovirus GII, enterotoxigenic E. coli, and Cryptosporidium spp. A considerable proportion of the disease in these children could not be explained by the pathogens tested. Severe GE cases associated with rotavirus and Shigella spp. were more likely to have a longer duration of vomiting and diarrhea, respectively. Cases attributed to Cryptosporidium spp. were more severe and required hospitalization. In the intention-to-treat population, VE was estimated to be 43.9% before and 46.5% after adjustment for co-infections; in the per-protocol population, VE was 46.7% before and 49.1% after adjustments. Rotavirus continued to be the leading cause of severe GE in this age group. The adjusted VE estimates obtained did not support co-infections as a major cause of lower vaccine performance in low- and middle-income countries.
Figures


References
-
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD, 2011. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 30: S1–S5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

