Neoadjuvant tislelizumab plus stereotactic body radiotherapy and adjuvant tislelizumab in early-stage resectable hepatocellular carcinoma: the Notable-HCC phase 1b trial
- PMID: 38627377
- PMCID: PMC11021407
- DOI: 10.1038/s41467-024-47420-3
Neoadjuvant tislelizumab plus stereotactic body radiotherapy and adjuvant tislelizumab in early-stage resectable hepatocellular carcinoma: the Notable-HCC phase 1b trial
Abstract
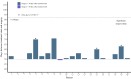
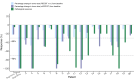
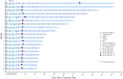
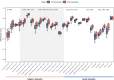
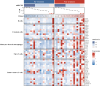
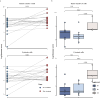
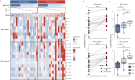
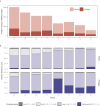
Notable-HCC (NCT05185531) is a phase 1b trial, aiming to evaluate the safety and preliminary effectiveness of neoadjuvant PD-1 blockade plus stereotactic body radiotherapy (SBRT) in early-stage resectable hepatocellular carcinoma (HCC). Twenty patients with HCC of BCLC stage 0-A received 3 Gy SBRT and two cycles of tislelizumab, an anti-PD-1 monoclonal antibody before the curative HCC resection. Primary endpoints were the surgery delay, radiographic and pathological tumor response after the neoadjuvant therapy, safety and tolerability. During the neoadjuvant therapy, treatment-related adverse events (TRAEs) of grade 1-2 occurred in all 20 patients (100%), eight patients (40%) had grade 3 TRAEs, no grade 4 to 5 TRAE occurred, and all resolved without corticosteroids treatment. Per mRECIST, the objective response rate was 63.2% (12/19), with 3 complete response; the disease control rate was 100%. Two (10.5%) patients achieved complete pathological response. No surgery delay occurred. The neoadjuvant therapy did not increase the surgical difficulty or the incidence of complications. Secondary endpoints of disease-free survival and overall survival were not mature at the time of the analysis. Our pilot trial shows that neoadjuvant therapy with anti-PD-1 + SBRT is safe and promotes tumor responses in early-stage resectable HCC.
© 2024. The Author(s).
Conflict of interest statement
L.Z. is on the speakers’ bureau for Bayer, MSD, AstraZeneca, Roche, BeiGene, Innovent, Junshi Biosciences, and Hengrui Medicine. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

