Ripretinib inhibits HIV-1 transcription through modulation of PI3K-AKT-mTOR
- PMID: 38627462
- PMCID: PMC11272926
- DOI: 10.1038/s41401-024-01282-z
Ripretinib inhibits HIV-1 transcription through modulation of PI3K-AKT-mTOR
Abstract
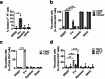
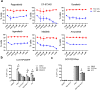
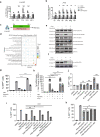
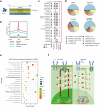
Despite the effectiveness of antiretroviral therapy (ART) in prolonging the lifespan of individuals infected with HIV-1, it does not offer a cure for acquired immunodeficiency syndrome (AIDS). The "block and lock" approach aims to maintain the provirus in a state of extended transcriptional arrest. By employing the "block and lock" strategy, researchers endeavor to impede disease progression by preventing viral rebound for an extended duration following patient stops receiving ART. The crux of this strategy lies in the utilization of latency-promoting agents (LPAs) that are suitable for impeding HIV-1 provirus transcription. However, previously documented LPAs exhibited limited efficacy in primary cells or samples obtained from patients, underscoring the significance of identifying novel LPAs that yield substantial outcomes. In this study, we performed high-throughput screening of FDA-approved compound library in the J-Lat A2 cell line to discover more efficacious LPAs. We discovered ripretinib being an LPA candidate, which was validated and observed to hinder proviral activation in cell models harboring latent infections, as well as CD4+ T cells derived from infected patients. We demonstrated that ripretinib effectively impeded proviral activation through inhibition of the PI3K-AKT-mTOR signaling pathway in the HIV-1 latent cells, thereby suppressing the opening states of cellular chromatin. The results of this research offer a promising drug candidate for the implementation of the "block and lock" strategy in the pursuit of an HIV-1 cure.
Keywords: HIV-1; PI3K-AKT-mTOR; block and lock; latency-promoting agents; ripretinib.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
BET degraders reveal BRD4 disruption of 7SK and P-TEFb is critical for effective reactivation of latent HIV in CD4+ T-cells.J Virol. 2025 Apr 15;99(4):e0177724. doi: 10.1128/jvi.01777-24. Epub 2025 Mar 11. J Virol. 2025. PMID: 40067013 Free PMC article.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article.
-
Inhibition of ALKBH5 demethylase of m6A pathway potentiates HIV-1 reactivation from latency.Virol J. 2025 Apr 28;22(1):124. doi: 10.1186/s12985-025-02744-4. Virol J. 2025. PMID: 40296171 Free PMC article.
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article.
-
Zinc finger protein 695 facilitates the proliferation of colorectal cancer cells through activation of the NEK2 and PI3K/Akt/mTOR signaling pathways.Oncol Rep. 2025 Oct;54(4):116. doi: 10.3892/or.2025.8949. Epub 2025 Jul 19. Oncol Rep. 2025. PMID: 40682845 Free PMC article.
Cited by
-
Pilaralisib inhibits the replication of enteroviruses by targeting the PI3K/AKT signaling pathway.Virol J. 2025 Jul 28;22(1):257. doi: 10.1186/s12985-025-02881-w. Virol J. 2025. PMID: 40717083 Free PMC article.
-
Paeoniflorin Inhibits Porcine Circovirus Type 2 Replication by Inhibiting Autophagy and Targeting AKT/mTOR Signaling.Vet Sci. 2025 Feb 2;12(2):117. doi: 10.3390/vetsci12020117. Vet Sci. 2025. PMID: 40005877 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

