Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism
- PMID: 38627524
- PMCID: PMC11186790
- DOI: 10.1038/s43587-024-00612-4
Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism
Abstract
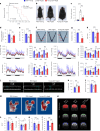
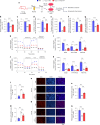
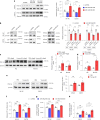
Recent investigations into heterochronic parabiosis have unveiled robust rejuvenating effects of young blood on aged tissues. However, the specific rejuvenating mechanisms remain incompletely elucidated. Here we demonstrate that small extracellular vesicles (sEVs) from the plasma of young mice counteract pre-existing aging at molecular, mitochondrial, cellular and physiological levels. Intravenous injection of young sEVs into aged mice extends their lifespan, mitigates senescent phenotypes and ameliorates age-associated functional declines in multiple tissues. Quantitative proteomic analyses identified substantial alterations in the proteomes of aged tissues after young sEV treatment, and these changes are closely associated with metabolic processes. Mechanistic investigations reveal that young sEVs stimulate PGC-1α expression in vitro and in vivo through their miRNA cargoes, thereby improving mitochondrial functions and mitigating mitochondrial deficits in aged tissues. Overall, this study demonstrates that young sEVs reverse degenerative changes and age-related dysfunction, at least in part, by stimulating PGC-1α expression and enhancing mitochondrial energy metabolism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

