Assessment of a novel electrochemically deposited smart bioactive trabecular coating (SBTC®): a randomized controlled clinical trial
- PMID: 38627712
- PMCID: PMC11022491
- DOI: 10.1186/s13005-024-00426-0
Assessment of a novel electrochemically deposited smart bioactive trabecular coating (SBTC®): a randomized controlled clinical trial
Abstract
Objectives: A randomized controlled clinical trial of dental implants was conducted to compare the clinical properties of a novel electrochemically deposited calcium phosphate coating to those of a common marketed surface treatment.
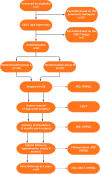
Material and methods: Forty implants of the same brand and type were placed in 20 fully edentulous participants requiring mandibular implantation. The two study groups were defined by the surface treatment of the implants. 20 implants in the control group were coated via a commercial electrochemical surface treatment that forms a mixture of brushite and hydroxyapatite, while the remaining 20 in the test group were coated with a novel electrochemical Smart Bioactive Trabecular Coating (SBTC®). A split-mouth design was employed, with each participants receiving one control implant in one mandibular side and a test implant in the other. To mitigate potential operator-handedness bias, control and test implants were randomly assigned to mandibular sides. All cases underwent digital planning, implant placement with a static surgical guide, and participants received locator-anchored full-arch dentures. The primary outcome was implant stability (measured using Osstell ISQ) assessed at insertion, loading, and then 3 months, 9 months, and 2 years post-insertion. The secondary outcome was bone level change (in millimeters) over the 2-year observation period. Oral health-related quality of life (OHRQL) was monitored using the OHIP-14 questionnaire. Complications and adverse events were recorded.
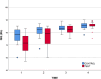
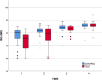
Results: Successful osseointegration and implant stability were achieved in all cases, allowing loading. ISQ values steadily increased throughout the observation period. While no significant differences were observed between the SBTC® and control coatings, the test group exhibited a higher ISQ gain. Bone resorption was somewhat lower in the SBTC® but not significantly so. Patients' OHRQL significantly improved after denture delivery and remained stable throughout the follow-up. No complications or adverse events were observed.
Conclusions: Based on the study results, we conclude that the new surface treatment is a safe alternative to the widely used control surface, demonstrating similar osseointegrative properties and time-dependent bone level changes. Further research may explore the broader implications of these findings.
Trial registration: The study is registered on clinicaltrials.gov under the identifier ID: NCT06034171.
Keywords: Dental implant; Hydroxyapatite; Osseointegration; RFA; Surface treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Immediate loading of dental implants supporting fixed partial dentures in the posterior mandible: a randomized controlled split-mouth study--machined versus titanium oxide implant surface.Int J Oral Maxillofac Implants. 2007 Jan-Feb;22(1):35-46. Int J Oral Maxillofac Implants. 2007. PMID: 17340895 Clinical Trial.
-
Immediate Vs Early Loading of Bone Level Tapered Dental Implants With Hydrophilic Surface in Rehabilitation of Fully Edentulous Maxilla: Clinical and Patient Centered Outcomes.J Oral Implantol. 2022 Oct 1;48(5):358-369. doi: 10.1563/aaid-joi-D-21-00045. J Oral Implantol. 2022. PMID: 34937085 Clinical Trial.
-
Report of a case receiving full-arch rehabilitation in both jaws using immediate implant loading protocols: a 1-year resonance frequency analysis follow-up.Clin Implant Dent Relat Res. 2006;8(1):25-31. doi: 10.2310/j.6480.2005.00027.x. Clin Implant Dent Relat Res. 2006. PMID: 16681490
-
Do Implant Coatings Affect Healing of Placed Implants? An Umbrella Review.Int J Oral Maxillofac Implants. 2024 Apr 24;39(2):206-223. doi: 10.11607/jomi.10492. Int J Oral Maxillofac Implants. 2024. PMID: 38657215
-
A systematic assessment of the stability of SLA® vs. SLActive® implant surfaces over 12 weeks.Evid Based Dent. 2025 Mar;26(1):67-68. doi: 10.1038/s41432-024-01097-1. Epub 2025 Jan 7. Evid Based Dent. 2025. PMID: 39775154 Free PMC article.
Cited by
-
A Comprehensive Review of the Contemporary Methods for Enhancing Osseointegration and the Antimicrobial Properties of Titanium Dental Implants.Cureus. 2024 Sep 5;16(9):e68720. doi: 10.7759/cureus.68720. eCollection 2024 Sep. Cureus. 2024. PMID: 39238921 Free PMC article. Review.
-
Efficacy of Biomimetic Hydroxyapatite in the Treatment of Extrinsic Dental Stains in Smokers and Non-Smokers.Materials (Basel). 2025 May 23;18(11):2441. doi: 10.3390/ma18112441. Materials (Basel). 2025. PMID: 40508439 Free PMC article.
References
-
- Elias CN. Factors affecting the success of dental implants. Implant dentistry: a rapidly evolving practice Rijeka: InTech. 2011: p. 319-64. https://www.intechopen.com/chapters/18426.
-
- Searson LJ. Gough M.Hemmings K. Implantology in General Dental Practice. Vol 4, Tokyo: QuintessencePublishing Company Limited; 2019:15-32.
-
- Sullivan RM. Implant dentistry and the concept of osseointegration: a historical perspective. J Calif Dent Assoc. 2001;29(11):737–744. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

